Acer's (ACER) NDA for ACER-001 in UCD Gets FDA Acceptance
Acer Therapeutics Inc. ACER, along with partner Relief Therapeutics Holding, announced that the FDA has accepted the new drug application ("NDA") for its investigational product candidate, ACER-001 (sodium phenylbutyrate), for the treatment of patients with urea cycle disorders (UCDs), a group of disorders caused by genetic mutations.
With the FDA accepting the NDA for review, a decision from the regulatory body is expected on Jun 5, 2022. The company had submitted the NDA in August 2021.
Shares of Acer were up 16.5% on Wednesday following the announcement of the news. The stock has rallied 10.7% so far this year against the industry’s decline of 18.7%.
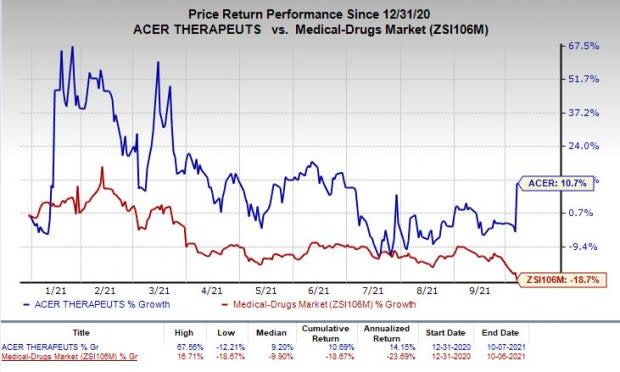
Image Source: Zacks Investment Research
The abovementioned NDA for ACER-001 was based on data from two bioequivalence studies in which treatment with ACER-001 demonstrated similar relative bioavailability for both phenylbutyrate and phenylacetate, the active metabolite of sodium phenylbutyrate, compared to Buphenyl (sodium phenylbutyrate).
We note that current treatments for UCDs include nitrogen scavengers, Ravicti and Buphenyl, both marketed by Horizon Therapeutics HZNP. Ravicti and Buphenyl became part of Horizon’s portfolio through the May 2015 Hyperion Therapeutics acquisition.
Acer plans to submit a marketing authorization application for ACER-001 for the treatment of patients with UCDs in Europe in the second or third quarter of 2022.
The company is developing ACER-001 for the treatment of various inborn errors of metabolism. Apart from UCDs, the candidate is also being developed for maple syrup urine disease.
We note that Acer has no approved product in its portfolio at the moment. Therefore, the successful development of ACER-001, along with other pipeline candidates, remains critical for the company’s long-term growth.
Zacks Rank & Stocks to Consider
Acer currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the same sector are Enlivex Therapeutics Ltd. ENLV and Avenue Therapeutics, Inc. ATXI, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Enlivex Therapeutics’ loss per share estimates have narrowed 14.8% for 2021 and 3.7% for 2022 over the past 60 days. The stock has soared 14.6% year to date.
Avenue Therapeutics’ loss per share estimates have narrowed 22.2% for 2021 and 73.6% for 2022 over the past 60 days.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Horizon Therapeutics Public Limited Company (HZNP) : Free Stock Analysis Report
Avenue Therapeutics, Inc. (ATXI) : Free Stock Analysis Report
Opexa Therapeutics, Inc. (ACER) : Free Stock Analysis Report
Enlivex Therapeutics Ltd. (ENLV) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 