Angion (ANGN) Kidney Drug Misses Third Study Goal in 2021
Angion ANGN and partner Vifor Pharma announced that the phase II GUARD study evaluating lead candidate, ANG-3777, in patients undergoing cardiac surgery involving cardiopulmonary bypass with risks of developing acute kidney injury (CSA-AKI) did not achieve the primary endpoint.
The GUARD study was designed to determine the feasibility of advancing ANG-3777 into a phase III study in patients having CSA-AKI, for which there are currently no approved therapies.
The GUARD study failed to achieve its primary endpoint of percentage increase in serum creatinine based on the area under the curve, with no significant difference in the short-term endpoint between ANG-3777 and placebo.
Nonetheless, the study did indicate a potential benefit in patients receiving ANG-3777 over those receiving placebo, based on the secondary endpoint of MAKE90. MAKE90 is a composite endpoint combining death, initiation of renal replacement therapy, or a greater than 25% decline in Estimated Glomerular Filtration Rate (eGFR) present 90 days after the surgery. Fewer patients in the ANG-3777 arm had a MAKE90 event compared to those in the placebo arm.
Based on results from this endpoint, Angion and Vifor Pharma will continue to review the data.
Angion also noted that the number of patients who experienced a decline in kidney function was lower in patients receiving ANG-3777 than patients receiving placebo.
Yet, the other secondary endpoints of the study including endpoints on MAKE30 and the incidence of AKI through Day 6 did not show a clinical benefit.
Shares of Angion declined 25.2% on Dec 10, following the announcement. In fact, the stock has plunged 84.7% so far this year in comparison with the industry’s 22.8% fall.
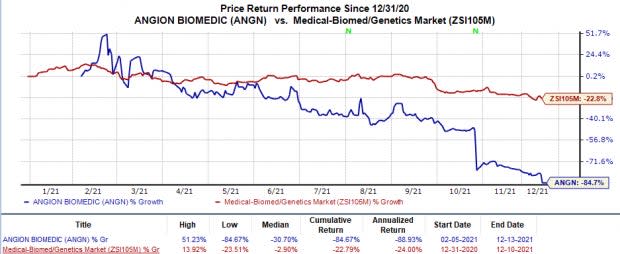
Image Source: Zacks Investment Research
Please note that Angion is developing ANG-3777 for renal indications in collaboration with Vifor Pharma. Last year in November, the company out-licensed global rights (excluding China, Taiwan, Hong Kong, and Macau) to Vifor to develop, manufacture, and commercialize ANG-3777 in all therapeutic, prophylactic, and diagnostic uses for renal indications, including forms of acute kidney injury, and congestive heart failure.
We inform investors that this is the third study involving ANG-3777, wherein it did not achieve its primary endpoint. Earlier this June, Angion reported that the phase II ALI-201 study evaluating ANG-3777 in patients with severe COVID-related pneumonia at high risks of acute respiratory distress syndrome failed to achieve primary or secondary efficacy endpoints. The company also reported in October that a phase III study evaluating AGN-3777 in kidney transplant patients who were at risk of developing delayed graft function failed to achieve a statistically significant difference from placebo on the primary endpoint of eGFR at 12 months.
Apart from ANG-3777, Angion is also advancing ANG-3070, a tyrosine kinase inhibitor, and inhibitor of rho kinase 2, in a phase II study in patients with primary proteinuric disease. The company expects to start enrolling patients in the study by fourth-quarter 2021.
Angion Biomedica Corp. Price
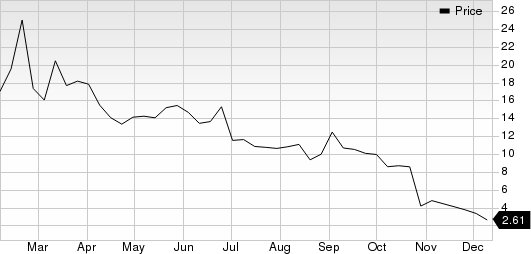
Angion Biomedica Corp. price | Angion Biomedica Corp. Quote
Zacks Rank & Other Stocks to Consider
Angion presently carries a Zacks Rank #2 (Buy). Other top-ranked stocks in the biotech/drug sector include Endo International ENDP, IVERIC bio ISEE and Precision BioSciences DTIL. While both Endo International and Precision BioSciences currently sport a Zacks Rank #1 (Strong Buy), IVERIC bio currently carries a Zacks Rank #2 at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Endo International’s earnings per share estimates for 2021 have increased from $2.29 to $2.84 in the past 60 days. The same for 2022 has increased from $2.24 to $2.47 in the past 60 days.
Earnings of Endo International beat estimates in all the last four quarters, with the average being 57.7%.
Precision BioSciences’ loss per share estimates for 2021 have narrowed from $1.17 to $0.65 in the past 60 days. The same for 2022 has narrowed from $2.39 to $1.91 in the past 60 days. Shares of Precision BioSciences have risen 2.9% in the year so far.
Earnings of Precision BioSciences beat estimates in all the last four quarters, delivering a surprise of 76.9%, on average.
IVERIC bio’s loss per share estimates for 2021 have narrowed from $1.18 to $1.09 in the past 60 days. The same for 2022 has narrowed from $1.17 to $1.03 in the past 60 days. Shares of IVERIC bio have gained 111.1% in the year so far.
Earnings of IVERIC bio missed estimates in three of the last four quarters and surpassed expectations once, with the negative surprise being 5.6%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Endo International plc (ENDP) : Free Stock Analysis Report
Angion Biomedica Corp. (ANGN) : Free Stock Analysis Report
Precision BioSciences, Inc. (DTIL) : Free Stock Analysis Report
IVERIC bio, Inc. (ISEE) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 