Annovis Bio’s Buntanetap Found Safe and Effective in High-Risk Alzheimer's Patients

Join Our Investor Call for In-Depth Findings Discussion
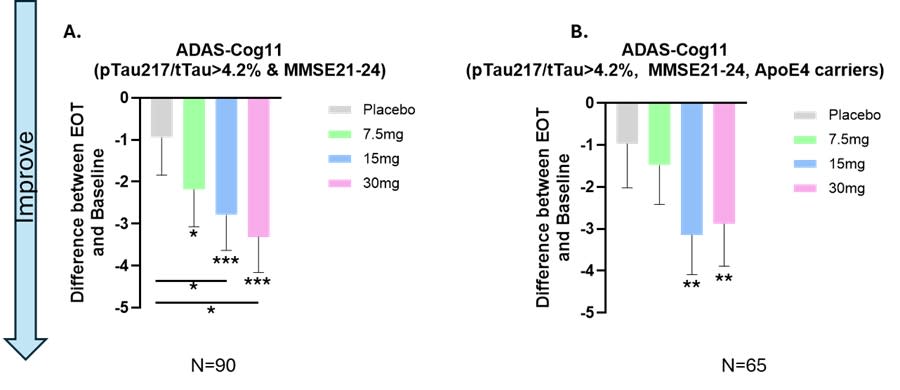
Efficacy in Early AD Patients
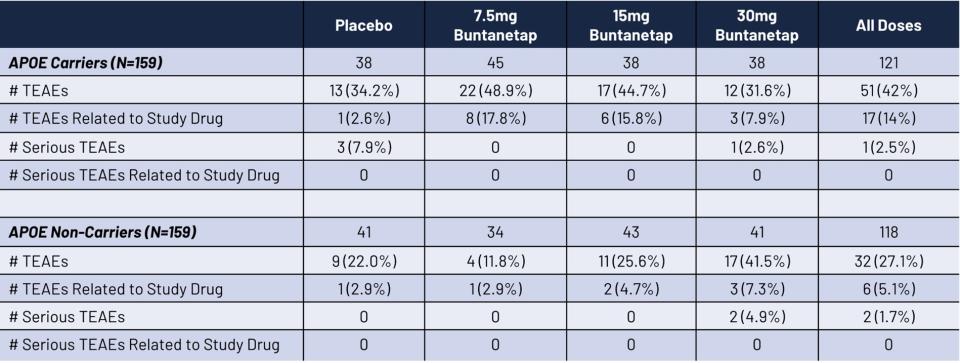
Comparable Safety
MALVERN, Pa., June 11, 2024 (GLOBE NEWSWIRE) -- via IBN – Annovis Bio Inc. (NYSE: ANVS) (“Annovis” or the “Company”), a late-stage drug platform company developing novel therapies for neurodegenerative diseases such as Alzheimer’s (AD) and Parkinson’s disease (PD), today announces that its recent Phase II/III Alzheimer’s study of its lead drug candidate, Buntanetap, showed statistically significant efficacy and safety in both carriers and non-carriers of Apolipoprotein E4 (APOE4), a genetic cause of AD.
Interested parties are encouraged to register for the upcoming investor call today at 4:30 PM ET, where detailed findings will be discussed. https://zoom.us/webinar/register/3117176913600/WN_Ev_1s7l2RUKmIQJNdko5iA
Key Findings:
Efficacy in Early AD Patients: In patients with early AD (MMSE 21-24), Buntanetap showed a statistically significant dose-response in ADAS-Cog11 scores, with a -3.3 points improvement over baseline and -2.3 points improvement from placebo.
APOE4 Carriers: In APOE4 carriers treated with 15mg Buntanetap, there was a -3.15 improvement in ADAS-Cog11 scores.
Comparable Safety: Buntanetap was found to be equally safe in both APOE4 carriers and non-carriers, with no instances of ARIA (Amyloid-Related Imaging Abnormalities).
Patient Breakdown: The study included 159 APOE4 carriers (33 homozygotes and 126 heterozygotes) and 159 APOE4 non-carriers.
Scientific Context: Recent findings published in Nature Medicine have redefined APOE4 homozygosity as a distinct genetic form of Alzheimer’s disease, requiring individualized prevention strategies, clinical trials, and treatments. This study emphasized the near-full penetrance of AD biology in APOE4 homozygotes, suggesting that these patients represent a significant target group for therapeutic interventions.
Safety Insights: Dr. Samuel Gandy, an Alzheimer’s researcher at Mount Sinai, highlighted the heightened safety risks for APOE4 homozygotes from anti-amyloid drugs, such as Leqembi, which have been associated with serious side effects like brain swelling and bleeding. When the Food and Drug Administration approved the anti-amyloid drug Leqembi last year, it required a black-box warning — the agency’s strongest caution — because of safety concerns for people with two copies of APOE4. However, Buntanetap demonstrated no increased safety issues compared to placebo, even in APOE4 carriers.
During our upcoming investor call, we will discuss the recent New York Times article that underscores the serious implications for APOE4 carriers.
Future Plans: Encouraged by these results, Annovis Bio is planning an 18-month Phase III trial focusing on biomarker-positive early AD patients. This trial aims to further validate Buntanetap’s efficacy and safety profile and will be conducted under the guidance of the FDA.
Investor Call: Annovis Bio will host an investor call to discuss these findings in detail and outline the future development plans for Buntanetap.
Date and Time: June 11, 2024, 4:30 pm ET.
Register Now: https://zoom.us/webinar/register/3117176913600/WN_Ev_1s7l2RUKmIQJNdko5iA
About Buntanetap: Buntanetap (formerly known as Posiphen or ANVS401) targets neurodegeneration by inhibiting the formation of multiple neurotoxic proteins, including amyloid beta, tau, alpha-synuclein, and TDP43. By improving synaptic transmission, axonal transport, and reducing neuroinflammation, Buntanetap aims to reverse neurodegeneration in AD, PD, and other neurodegenerative diseases.
About Annovis Bio Inc.: Headquartered in Malvern, Pennsylvania, Annovis Bio Inc. is dedicated to addressing neurodegeneration in diseases such as AD and PD. The company’s innovative approach targets multiple neurotoxic proteins, aiming to restore brain function and improve the quality of life for patients. For more information, visit www.annovisbio.com and follow us on LinkedIn and X.
Forward-Looking Statements
This press release contains "forward-looking" statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements include, but are not limited to, the Company's plans related to clinical trials. Forward-looking statements are based on current expectations and assumptions and are subject to risks and uncertainties that could cause actual results to differ materially from those projected. Such risks and uncertainties include, but are not limited to, those related to patient enrollment, the effectiveness of Buntanetap, and the timing, effectiveness, and anticipated results of the Company's clinical trials evaluating the efficacy, safety, and tolerability of Buntanetap. Additional risk factors are detailed in the Company's periodic filings with the SEC, including those listed in the "Risk Factors" section of the Company's Annual Report on Form 10-K and Quarterly Reports on Form 10-Q. All forward-looking statements in this press release are based on information available to the Company as of the date of this release. The Company expressly disclaims any obligation to update or revise its forward-looking statements, whether as a result of new information, future events, or otherwise, except as required by law.
Contacts
Annovis Bio, Inc.
101 Lindenwood Drive
Suite 225
Malvern, PA 19355
www.annovisbio.com
Investor Contact
Scott McGowan
InvestorBrandNetwork (IBN)
Phone: 310.299.1717
IR@annovisbio.com
Investor Website
Attachments


 Yahoo Finance
Yahoo Finance 