Here's How Alnylam Pharmaceuticals, Inc. Crushed It in 2017
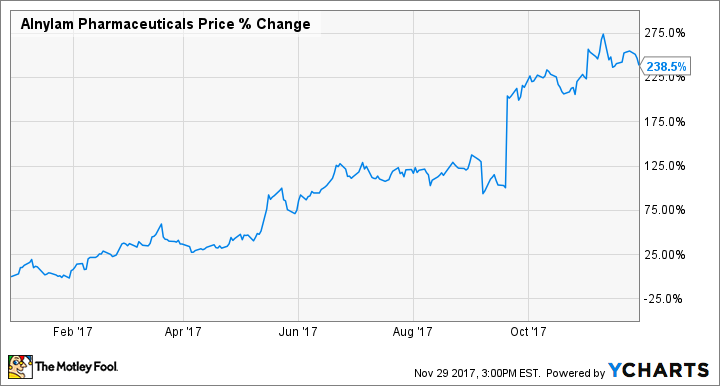
After a horrible 2016 following the loss of its lead drug candidate, Alnylam Pharmaceuticals (NASDAQ: ALNY) has had a pretty stellar year, up 238% so far.
Much of that increase has come in the last few months after Alnylam and its partner, Sanofi (NYSE: SNY), announced that Alnylam's new lead drug, patisiran, which treats hereditary ATTR amyloidosis (hATTR), passed the phase 3 Apollo trial. A few months later, the companies presented details of the trial at a medical meeting, demonstrating how much the drug helped patients compared to placebo.
Good data, but is it better than the competition?
Specifically, patisiran improved the Modified Neuropathy Impairment Score (mNIS+7) by 34.0 points compared to placebo after 18 months of treatment. Patients taking placebo worsened while those taking patisiran improved their mNIS+7 score by 6.0 points. You can't ask for much more than that.
Unfortunately, Alnylam and Sanofi have competition from Ionis Pharmaceuticals' (NASDAQ: IONS) inotersen, which also treats hATTR. In its phase 3 trial, inotersen improved the mNIS+7 score by 19.73 points compared to placebo, which isn't as good as patisiran's 30-point benefit, but Ionis took the measurement at 15 months, shorter than the 18-month reading for patisiran. Ionis also has data showing its drug helps patients as early as eight months, with an 8.69-point benefit compared to placebo.
Barring some manufacturing issues, it seems likely that both drugs will be approved next year, and it will come down to marketing, which at the moment could benefit Alnylam since it has a large-pharma partner to sell the drug outside of the U.S., Canada, and Western Europe. Ionis recently regained full rights to inotersen from its former partner, GlaxoSmithKline (NYSE: GSK), and has indicated it's looking for a new partner but could launch on its own.

Image source: Getty Images.
Rest of the pipeline progressing
Beyond patisiran, Alnylam has three other drugs in phase 3 development that are progressing along, albeit somewhat slowly as is often the case with drug development.
Inclisiran, a cholesterol-lowering drug Alnylam is developing with The Medicines Company (NASDAQ: MDCO), is in a rather large phase 3 program with four clinical trials, so data won't be available for a while. Alnylam and The Medicines Company are shooting for submitting marketing applications toward the end of 2019.
Fitusiran, which treats hemophilia, hit a snag when a patient died in the clinical trial, but Alnylam and the Food and Drug Administration worked out protocol changes for the clinical trial to increase safety that will allow the company to restart the program later this year.
Givosiran, which treats an orphan disease called acute hepatic porphyrias, recently entered a phase 3 trial called Envision. Of the three dugs, givosiran could be first to market since an interim peek at the data, which could be enough to gain FDA approval, is expected in the middle of next year.
Further back in the pipeline, Alnylam has three earlier-stage drugs, including a follow-on to patisiran, ALN-TTRsc02, which could be dosed as infrequently as every three to six months, making it substantially more convenient than patisiran and Ionis' inotersen.
Looking forward
The share price increase over the last year implies that investors have already declared patisiran the winner in the hATTR market, so to keep the momentum going, Alnylam will need to hit the ground running once patisiran is approved next year.
Positive interim results for givosiran could also give shares a boost next year. An approval probably wouldn't come until 2019, but the market is forward-looking and should price in sales if it's clear from the data that the drug is likely to be approved by regulators.
More From The Motley Fool
6 Years Later, 6 Charts That Show How Far Apple, Inc. Has Come Since Steve Jobs' Passing
Why You're Smart to Buy Shopify Inc. (US) -- Despite Citron's Report
Brian Orelli has no position in any of the stocks mentioned. The Motley Fool owns shares of and recommends Alnylam Pharmaceuticals and Ionis Pharmaceuticals. The Motley Fool has a disclosure policy.

 Yahoo Finance
Yahoo Finance