AstraZeneca shares fall on vaccine disappointment
Live updates: FTSE rises as vaccine optimism boosts markets
Analysis: How AstraZeneca went from takeover target to FTSE winner
Shares in AstraZeneca closed down almost 4pc as investors were left disappointed by an update on its coronavirus vaccine trial and one analyst claimed the vaccine would not win approval from US regulators.
A progress report on the clinical trial, which is still ongoing, suggests the vaccine being trialled with Oxford University is 70pc effective on average in preventing coronavirus. This is less than the 90pc efficacy rates reported by US vaccine hopefuls Moderna and Pfizer over the past two weeks.
Investors were also spooked after a US-based analyst questioned the trial results in a note to investors and said the vaccine would not get the FDA nod.
However, other analysts were quick to rebut his claims, saying it was too early to make a call and that much clinical trial data on all three vaccines still needed to be analysed.
“Far be it from me to dispute one of my peers or competitors but I think it’s too early for me or anyone, in fact, to be making a statement on regulatory approval,” said one analyst. “None of us have seen the full data for one, let alone all trials. Comparing apples and pears at this stage is a little misleading.”
Asked about the note, Ruud Dobber, head of AstraZeneca’s biopharmaceuticals business unit, told Bloomberg TV: “I think it’s far too early to speculate about how regulators will react.”
AstraZeneca said the vaccine had no serious side effects and that trial had shown a smaller dose, followed by a full dose, had an efficacy rate of 90pc.
Another analyst said: “I think everyone just wants to see more data from Astra to confirm what exactly is going to happen in terms of the different dosing regimens. We could potentially have a very effective, safe vaccine that is more easily mass produced and distributed than Pfizer or Moderna’s.”
Unlike those from Pfizer and Moderna, Astra's vaccine can be stored in a refrigerator for up to six months.
AstraZeneca expects to be able to make 3bn doses by the end of next year, compared with Pfizer’s 1.3bn and Moderna’s target of between 500m and 1bn.
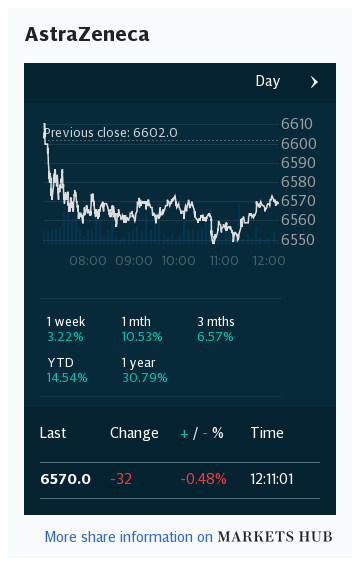
Shares in AstraZeneca closed the day down 3.8pc at £80, valuing the company at £104bn – second only to Unilever on the FTSE 100.
Meanwhile, Moderna’s chief executive, Stephane Bancel, sold another $1.4m (£1.04m) worth of shares in the US biotech. Mr Bancel’s net worth is $3.1bn, according to the Bloomberg Billionaires Index.
Read more: Passengers will need vaccine proof for international flights, warns Qantas boss

 Yahoo Finance
Yahoo Finance 