Attention Biotech Investors: Keep Your Date With July PDUFA Action-Days

June was event-filled as far as the PDUFA calendar was concerned. The month ushered in good tidings for the biotech sector, as most applications for approval pending before the FDA were favorably viewed.
The iShares NASDAQ Biotechnology Index (ETF) (NASDAQ: IBB) has been up 11.70 percent thus far this month compared to a 12.39-percent gain for the NYSE ARCA Biotech Index.
Benzinga took a look at what is in store for the sector in the upcoming month.
A June Recap
Recapping the approvals in June:
New Molecular Entities, or NME, approvals have increased to 22 thus far this year, with two molecules receiving approval in June:
1. Portola Pharmaceuticals Inc (NASDAQ: PTLA)'s Bevyxxa was approved for treating venous thromoboembolism in adult patients hospitalized for an acute medical illness.
2. Privately-held Melinta Therapeutics announced FDA approval for Baxdela for acute bacterial skin and structure infection.
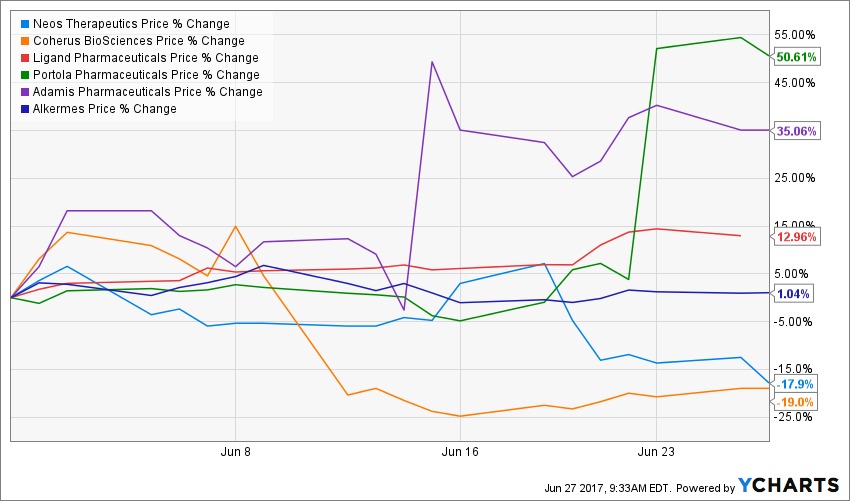
NEOS
Source: Y Charts
1. Ocular Looks To Bulls Eye After Failure.
On July 25, 2016, the FDA issued a CRL for Dextenza, citing deficiencies in manufacturing process and controls identified during a pre-NDA approval inspection of the manufacturing facility. On Feb. 22, 2017, the FDA accepted the company's resubmission.
Source: Ocular Therapeutics.
2. Puma Biotechnology's Leap Of Faith In Breast Cancer Therapy.
The company announced FDA acceptance of its NDA filing on Sept. 20, 2016, with the PDUFA date estimated to be July 21, 2017. An FDA Advisory Committee meeting held on May 24, 2017, voted 12–4 in favor of recommending approval.
3. GlaxoSmothKline's Lupus Drug Up For Approval.
Benlysta is a human monoclonal antibody currently licensed for use intravenously as a one-hour infusion every four weeks. The BLA filing completed on Sept. 23, 2016, was based on results from the BLISS-SC Phase III pivotal study, which evaluated belimumab 200mg administered weekly via subcutaneous injection plus standard of care.
Source: What-when-how
4. Eagle Looks To Strike It Rich With Heat Stroke Drug.
The FDA granted priority review status for the treatment candidate on March 27, 2017.
"There is currently no approved pharmacological treatment for EHS. If Ryanodex is approved, Eagle will be the first to market with a potentially transformational therapy. EHS can strike anyone, but athletes, our military and outdoor workers are especially vulnerable. We look forward to working with the FDA throughout the review process and to their expedited decision in July 2017," said Scott Tarriff, CEO of Eagle, in the release announcing its priority review status.
Related News:
Is It Time To Step To The Sidelines On Regeneron?
Bellicum Pharmaceuticals EHA Presentation Helps Validate Its Platform

See more from Benzinga
© 2017 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.

 Yahoo Finance
Yahoo Finance