Biotech Stock Roundup: GILD, BMY's Regulatory News, BLUE's Therapy Approval & More
It has been a busy week for the biotech companies with a plethora of important updates. Drug approvals and other regulatory news grabbed the spotlight this week.
Recap of the Week’s Most Important Stories:
Regulatory Updates From Gilead: Gilead Sciences, Inc. GILD announced that its antiviral treatment Veklury (remdesivir) is now recommended by the World Health Organization (“WHO”) to treat patients with severe COVID-19. The organization also continues to conditionally recommend Veklury for those with non-severe COVID-19 at the highest risk of hospitalization. This conditional recommendation was based on the final results of the WHO-sponsored SOLIDARITY study. The trial showed a statistically significant 17% lower relative risk of death or progression to needing ventilation in patients requiring supplemental oxygen at baseline compared to the standard of care.
In addition, Gilead announced that the Committee for Medicinal Products for Human Use (“CHMP”) of the European Medicines Agency (“EMA”) adopted a positive opinion to extend the indication of Veklury. The CHMP recommended Veklury for treating pediatric patients (weighing at least 40 kg) who do not require supplemental oxygen and are at increased risk of progressing to severe COVID-19 and pediatric patients with SARS-CoV-2 with pneumonia who require supplemental oxygen.
Concurrently, the CHMP issued a positive opinion for Chimeric Antigen Receptor (CAR) T-cell therapy Yescarta (axicabtagene ciloleucel) for adult patients with diffuse large B-cell lymphoma (DLBCL) and high-grade B-cell lymphoma (HGBL) that relapses within 12 months from completion of, or is refractory to, first-line chemoimmunotherapy. A final decision is expected in the coming months.
Gilead currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Updates From Bristol Myers: Bristol Myers Squibb BMY announced that the European Commission (“EC”) has approved its skin cancer drug Opdualag for the first-line treatment of advanced (unresectable or metastatic) melanoma in adults and adolescents 12 years of age and older with tumor cell PD-L1 expression <1%. Opdualag is a first-in-class, fixed-dose dual immunotherapy combination treatment of the PD-1 inhibitor Opdivo (nivolumab) and novel LAG-3-blocking antibody relatlimab.
Bristol Myers also announced positive results from the phase III CheckMate-76K study, currently evaluating its blockbuster immuno-oncology drug Opdivo as a single agent in the adjuvant setting in patients with completely resected stage IIB/C melanoma, a form of skin cancer. Results showed that the study met its primary endpoint and demonstrated a statistically significant and clinically meaningful benefit in recurrence-free survival (RFS) versus placebo at a pre-specified interim analysis. The secondary endpoints of the trial include overall survival (OS), distant metastases-free survival (DMFS), progression-free survival on next-line therapy (PFS2) and safety endpoints. Shares of the company gained on these results.
bluebird bio Gets Approval for Second Product: bluebird bio BLUE obtained accelerated approval from the FDA for its gene therapy, elivaldogene autotemcel, under the brand name Skysona to slow the progression of neurologic dysfunction in boys 4-17 years of age with early, active cerebral adrenoleukodystrophy (CALD). The biologics license application (BLA) was reviewed by the FDA under Priority Review, and bluebird received a rare pediatric priority review voucher upon approval.
The regulatory body also lifted the clinical hold that was put in place in August 2021, prior to the completion of its review of the BLA. The approval was based on bluebird bio’s phase II/III study ALD-102 (starbeam) and a phase III ALD-104 (N=35) study. The accelerated approval of Skysona is based on 24-month Major Functional Disabilities (MFD)-free survival.
Eloxx Plunges on Study Update: Shares of Eloxx Pharmaceuticals, Inc. ELOX plunged after the company announced disappointing top-line results from the phase II study of investigational pipeline candidate ELX-02 in combination with Vertex Pharmaceuticals’ ivacaftor in Class 1 cystic fibrosis (CF) patients with at least one nonsense mutation. The phase II study assessed the safety and biological activity of ELX-02 in G542X nonsense mutation Class 1 CF patients as monotherapy and in combination with ivacaftor.
Though the drug was well-tolerated in study participants, the study failed to achieve statistical significance for efficacy endpoints, including changes from baseline in sweat chloride concentration (SCC) and percent forced expiratory volume (FEV1) in class 1 CF patients. Investors were disappointed as ELX-02 is Eloxx’s most advanced pipeline candidate and study failure puts a question mark on the company’s growth prospects.
Alnylam’s Drug Approval: Alnylam Pharmaceuticals, Inc. ALNY announced that the European Commission has granted marketing authorization to Amvuttra (vutrisiran), an RNAi therapeutic for the treatment of hereditary transthyretin-mediated (hATTR) amyloidosis in adult patients with stage 1 or stage 2 polyneuropathy. The approval is based on positive 18-month results from the HELIOS-A Phase 3 study, where the drug significantly improved the signs and symptoms of hATTR amyloidosis, with more than 50% of patients experiencing halting or reversal of their polyneuropathy manifestations. The drug was also approved by the FDA in June 2022. The approval strengthens Alnylam’s portfolio, which has a deep pipeline of investigational medicines, including six product candidates in late-stage development.
Performance
The Nasdaq Biotechnology Index has lost 4.77% in the past five trading sessions. Among the biotech giants, Moderna has lost 9.78% during the period. Over the past six months, shares of Vertex have soared 12.04%. (See the last biotech stock roundup here: Biotech Stock Roundup: BMY Surges on Drug Approval, REGN Up on Update & More)
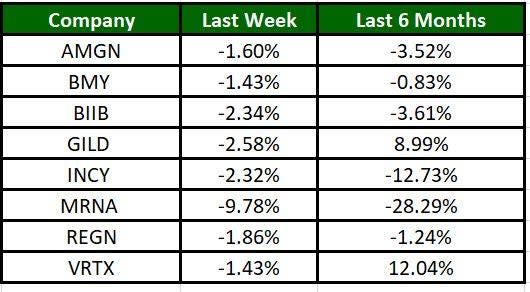
Image Source: Zacks Investment Research
What's Next in Biotech?
Stay tuned for other updates.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Alnylam Pharmaceuticals, Inc. (ALNY) : Free Stock Analysis Report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
bluebird bio, Inc. (BLUE) : Free Stock Analysis Report
Senesco Technologies Inc. (ELOX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 