bluebird (BLUE) Tops on Q1 Sales, Zynteglo Progresses Well
bluebird bio BLUE registered revenues of $18.6 million in the first quarter, up from $2.4 million recorded in the year-ago quarter and beating the Zacks Consensus Estimate of $18 million.
The $16.2-million upside can be attributed to increased Zynteglo product revenues.
The FDA approved Zynteglo for the treatment of beta-thalassemia in adult and pediatric patients requiring regular red blood cell transfusion on Aug 17, 2022, and Skysona for treating early, active cerebral adrenoleukodystrophy (CALD) on Sept 16, 2022.
In December 2023, the FDA approved its third gene therapy, lovotibeglogene autotemcel (lovo-cel), under the brand name Lyfgenia, for the treatment of sickle cell disease (SCD) in patients aged 12 years and older with a history of vaso-occlusive events.
Shares of the company have gained 14.9% in response to positive first-quarter results.
Shares of BLUE have plunged 18.8% in the year so far compared with the industry’s 6.3% decline.
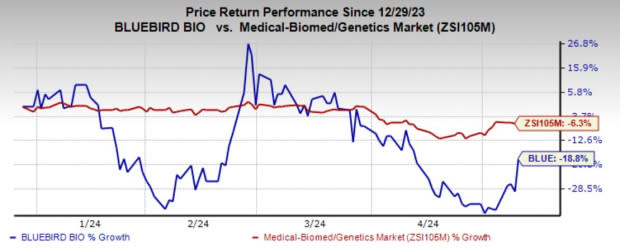
Image Source: Zacks Investment Research
As of Mar 31, 2024, bluebird had cash, cash equivalents and restricted cash balance of approximately $264 million.
Earlier in 2024, Bluebird Bio announced that it had received a $175 million five-year term loan facility from Hercules Capital, Inc. HTGC. The funding will extend the company’s cash runway by two years.
Per the agreement, bluebird will get the term loans in four tranches. The first tranche of $75 million was drawn upon the closing of the transaction. BLUE will be eligible to draw two additional tranches of $25 million each, subject to the achievement of commercial milestones.
BLUE expects its cash and cash equivalents, excluding restricted cash and assuming the remaining two tranches totaling $50 million in proceeds from its term loan facility with Hercules Capital are executed, will be sufficient for its planned operating expenses and capital expenditure requirements through the first quarter of 2026.
The agreement also states that a fourth tranche of up to $50 million may be available at the sole discretion of Hercules.
The company is restating its financial statements for 2022 and for the first three quarters of both 2022 and 2023.
Other Updates
bluebird also disclosed that 14 patient starts completed for Zynteglo and Skysona year to date (11 Zynteglo and 3 Skysona).
The first patient start for Lyfgenia was completed this month.
bluebird activated 64 qualified treatment centers (QTC) networks for Lyfgenia and Zynteglo. Six centers are also activated to administer Skysona for patients with CALD.
2024 Guidance
bluebird anticipates 85 to 105 patient starts (cell collections) combined across all three of its FDA-approved therapies (Lyfgenia, Zynteglo, Skysona) in 2024.
Gross-to-net discounts across all three products are expected to be in the range of 20-25% of gross revenue in 2024.
bluebird anticipates recognizing revenue from its first infusion of Lyfgenia in the third quarter of 2024.
Our Take
While bluebird’s progress with its portfolio is encouraging, gaining market share in the challenging gene therapy field will be a daunting task.
While Lyfgenia approval is a positive, the label carries a boxed warning of hematologic malignancy which had disappointed investors earlier.
Hence, bluebird will need to monitor patients closely for evidence of malignancy through complete blood counts at least every six months and integration site analysis at months six, 12 and as warranted.
Moreover, Vertex VRTX and CRISPR Therapeutics’ CRSP CRISPR/Cas9 genome-edited cell therapy, Casgevy, will make it tough for Lyfgenia to gain inroads. Casgevy is also approved for SCD.
Casgevy is also approved for the treatment of transfusion-dependent beta thalassemia. Vertex leads the global development, manufacturing, regulatory and commercialization of Casgevy with support from CRSP.
BLUE stated that it has successfully confirmed prior authorization approval for commercial and Medicaid-insured patients for Lyfgenia.
bluebird currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
Hercules Capital, Inc. (HTGC) : Free Stock Analysis Report
bluebird bio, Inc. (BLUE) : Free Stock Analysis Report
CRISPR Therapeutics AG (CRSP) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 