Bristol-Myers Posts Data on Empliciti, China Approves Opdivo
Bristol-Myers Squibb Company BMY announced positive results from the phase II study, ELOQUENT-3, on oncology drug, Empliciti.
The study is evaluating the addition of Empliciti to Pomalyst and low-dose dexamethasone (EPd) in patients with relapsed/refractory multiple myeloma (RRMM). The international study is evaluating Pomalyst-based triplet combination in patients with RRMM, who received at least two prior therapies, including Revlimid and a proteasome inhibitor.
The study achieved its primary endpoint as the results show a statistically significant and clinically meaningful improvement in progression-free survival (PFS) for patients treated with EPd compared with only Pomalyst and dexamethasone (Pd).
The data were also presented at the 23rd Congress of the European Hematology Association.
Data from the study showed that patients randomized to EPd experienced a 46% reduction in risk of disease progression compared with patients randomized to Pd alone, with median PFS of 10.3 months compared with 4.7 months in Pd patients.
Additionally, the PFS benefit experienced among patients randomized to EPd was consistent among patients who had received two to three prior lines of therapy and four or more prior lines of therapy.
The combination therapy, if approved, can provide an important treatment option for patients with relapsed/refractory multiple myeloma whose disease has progressed after treatment with lenalidomide and a proteasome inhibitor.
Bristol-Myers is co-developing Empliciti with AbbVie ABBV.
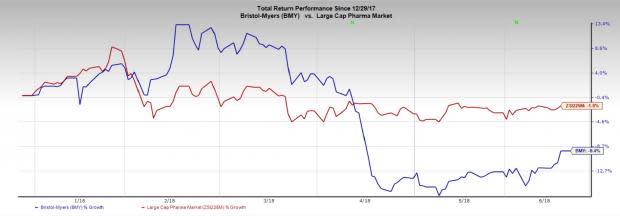
Bristol-Myers’ shares have declined 9.4% year to date compared with the industry’s decline of 1.8%.
Earlier, the company announced that the China National Drug Administration has approved its blockbuster immuno-oncology drug Opdivo, for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) after prior platinum-based chemotherapy in adult patients without EGFR or ALK genomic tumor aberrations.
Per the company, this is the first and only PD-1 inhibitor approved in China. The approval was based on data from the pivotal phase III study, CheckMate-078 trial, in which 90% of the patients enrolled were from China.
We note that Opdivo became the first PD-1 immune checkpoint inhibitor to gain regulatory approval. It is currently approved in several countries including the United States, the EU and Japan for several cancer indications.
Opdivo became the first PD-1 inhibitor to be approved for a hematological malignancy — classical Hodgkin lymphoma — in both the United States (May 2016) and the EU (November 2016). The drug has been performing impressively, due to demand resulting from the rapid commercial acceptance for several indications, including melanoma, renal cell carcinoma and second-line NSCLC.
Label expansion into additional indications would give the product access to a higher patient population and increase the commercial potential of the drug significantly.
However, Opdivo faces stiff competition from Merck’s MRK Keytruda and Roche’s RHHBY Tecentriq which can limit market share gains.
Zacks Rank & Another Stock to Consider
Bristol-Myers carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 (Strong Buy) Rank stocks here.
Today's Stocks from Zacks' Hottest Strategies
It's hard to believe, even for us at Zacks. But while the market gained +21.9% in 2017, our top stock-picking screens have returned +115.0%, +109.3%, +104.9%, +98.6%, and +67.1%.
And this outperformance has not just been a recent phenomenon. Over the years it has been remarkably consistent. From 2000 - 2017, the composite yearly average gain for these strategies has beaten the market more than 19X over. Maybe even more remarkable is the fact that we're willing to share their latest stocks with you without cost or obligation.
See Them Free>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 
