Cassava (SAVA) Begins First Efficacy Study on Simufilam in AD
Cassava Sciences, Inc. SAVA announced that it has initiated the first initial phase III efficacy study evaluating its investigational drug candidate, simufilam, for the treatment of patients with Alzheimer’s disease (“AD”).
The first double-blind, placebo-controlled phase III study, RETHINK-ALZ, will evaluate the safety and efficacy of simufilam (100 mg) in enhancing cognition and slowing cognitive and functional decline, over a period of 52 weeks. The study will enroll around 750 patients with mild-to-moderate AD in the United States, Canada and other countries worldwide.
The second double-blind, placebo-controlled phase III study, REFOCUS-ALZ, will evaluate the safety and efficacy of simufilam (100 mg and 50 mg) over a period of 78 weeks. The study is expected to begin by 2021-end and will enroll around 1000 patients with mild-to-moderate AD.
We note that both the above phase III efficacy studies are being conducted under the FDA’s Special Protocol Assessments (“SPA”). In August 2021, Cassava reached an agreement with the FDA on SPA for its phase III studies of simufilam for the treatment of AD.
Shares of Cassava have skyrocketed 670% so far this year against the industry’s decrease of 16.7%.
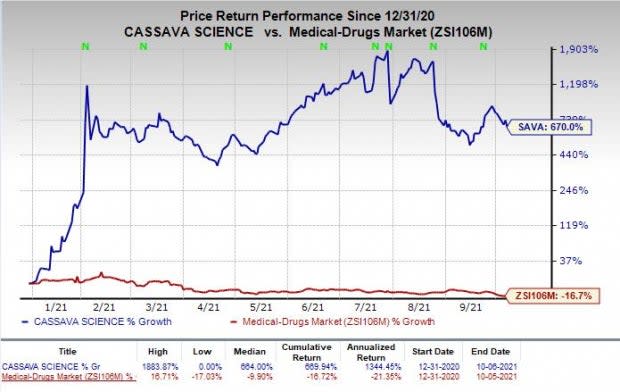
Image Source: Zacks Investment Research
Last month, Cassava announced top-line data from an interim analysis of the ongoing study on simufilam for treating patients with mild-to-moderate AD. Data from the same showed that the first 50 subjects who completed one year of open-label treatment with simufilam saw their cognition scores improve at an average of 3.2 points on the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) from the baseline.
Simufilam is a proprietary, small-molecule (oral) drug that restores the normal shape and function of altered filamin A, a scaffolding protein, in the brain.
We note that, the FDA approval of Biogen’s BIIB AD drug, Aduhelm (aducanumab), has put the spotlight on this promising yet challenging space. In June 2021, Biogen and its partner Eisai won the FDA approval for Aduhelm as the first and only AD treatment, after a few setbacks.
Some other companies are also working hard to get their AD drugs approved. Eli Lilly LLY is developing investigational antibody therapy, donanemab, for AD. Annovis Bio, Inc. ANVS is also developing its pipeline candidate, currently in a mid-stage study, for treating AD.
Zacks Rank
Cassava currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Cassava Sciences, Inc. (SAVA) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 