Coronavirus: AstraZeneca to start COVID-19 drug trial

Pharmaceuticals giant AstraZeneca (AZN.L) said on Tuesday it would start a new clinical trial of Calquence aimed at assessing it as a treatment for COVID-19.
The company said it hopes the drug will treat the exaggerated immune response associated with COVID-19 infections. Calquence is currently used to treat certain types of blood cancers.
AstraZeneca said “early clinical data” suggests Calquence “appears to reduce the severity of COVID-19-induced respiratory distress”.
The goal of the new trial, called CALVARI, is to assess the effectiveness and safety of the drug for patients afflicted with the novel coronavirus. If the trial is successful, AstraZeneca hopes the drug could help reduce mortality rates and the need for assisted ventilation.
“With this trial we are responding to the novel insights of the scientific community and hope to demonstrate that adding Calquence to best supportive care reduces the need to place patients on ventilators and improves their chances of survival,” said José Baselga, AstraZeneca’s executive vice president for oncology research and development.
“This is the fastest launch of any clinical trial in the history of AstraZeneca.”
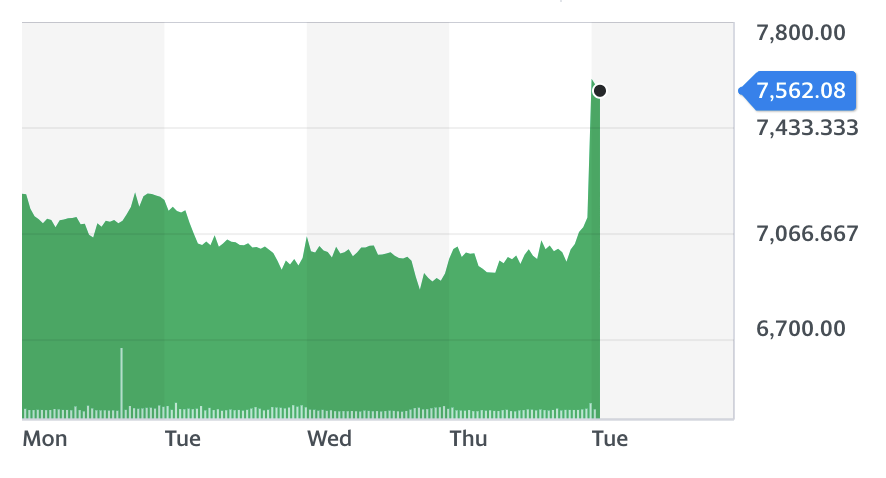
AstraZeneca shares jumped over 6% in the market open on the news.
Calquence is an inhibitor of the enzyme Bruton Tyrosine Kinase (BTK), which is a key part of the B-cell receptor signalling pathway.
“Given the well documented role of the protein BTK in regulating inflammation, it is possible that inhibiting BTK with acalabrutinib could provide clinical benefit in patients with advanced COVID-19 lung disease,” said Louis M. Staudt, M.D., Ph.D., chief of the Lymphoid Malignancies Branch at the National Cancer Institute (NCI).
“As with all new treatments, it will be necessary to gather data from clinical trials in order to understand the best and safest treatment options for patients.”
As of Tuesday, there were 1.9m confirmed cases of COVID-19 globally. Almost 120,000 people are confirmed to have died from the novel coronavirus.
Watch the latest videos from Yahoo UK

 Yahoo Finance
Yahoo Finance 
