Coronavirus: UK government in talks with Roche about ‘highly specific’ antibody tests
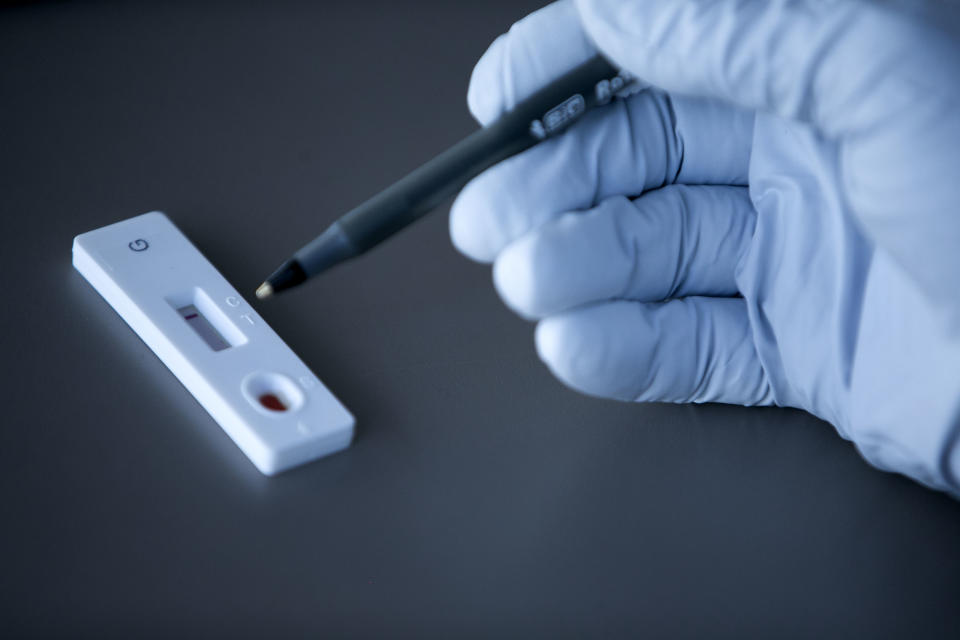
The UK government is holding talks with Swiss drugmaker Roche (ROG.SW) about an antibody test that is thought to be able to detect a previous coronavirus infection with 100% specificity.
Junior health minister Edward Argar said that the test had the potential to be a “game changer,” noting that a Public Health England (PHE) laboratory in Porton Down had determined that the test was accurate enough to use.
“We are now moving as fast as we can to discuss with Roche purchasing of those but I can’t give you an exact date when we’ll be able to start rolling them out,” Argar said.
Last month, the Roche test received approval from the European Union, passing the bloc’s conformity assessment. The US Food and Drug Administration gave the test an emergency use authorisation on 2 May.
READ MORE: Global stocks slide on rising US-China tensions and Fed warning
Governments are hoping that antibody tests could pave the way for a gradual reopening of their economies, since they can confirm that an already infected person has recovered from the virus.
However, the World Health Organisation has warned against governments issuing “immunity passports” on the basis of antibody tests, noting that there is “not enough evidence” that the presence of antibodies is indicative of immunity.
Some evidence suggests that antibodies develop soon after a coronavirus infection, but it is not clear whether that guarantees anything more than short-term immunity from reinfection.
John Newton, the national coordinator of the UK Coronavirus Testing Programme, nonetheless said that the Roche test was a “very positive development,” since a high specific antibody test was at least “a very reliable marker of past infection.”
Following a report in the Telegraph about the PHE approval, Roche confirmed in a statement late on Wednesday (13 May) that it was in discussions with the National Health Service about a phased roll-out of the test kits.
READ MORE: WH Smith sees 400% increase in online book sales
It said it would be in a position to provide hundreds of thousands of the kits each week, but noted that the test requires a blood sample to be taken by a qualified healthcare professional and processed in a laboratory.
Its high accuracy, Roche said, was “vitally important,” since viruses such as the common cold and SARS result in diseases that can produce very similar antibodies.
Watch the latest videos from Yahoo UK

 Yahoo Finance
Yahoo Finance 