CRISPR's (CRSP) CAR-T Cell Therapy Gets FDA's RMAT Designation
CRISPR Therapeutics AG CRSP announced that its wholly-owned allogeneic CAR-T cell therapy, CTX110, targeting CD19+ B-cell malignancies, has been granted Regenerative Medicine Advanced Therapy (“RMAT”) designation by the FDA.
The RMAT designation was created under the 21st Century Cures Act, and is granted to speed up the development and review of regenerative therapies that target serious or life-threatening conditions. The designation was bestowed on CTX110 due to its transformative potential for the treatment of hematological malignancies.
Shares of CRISPR Therapeutics have declined 47.4% so far this year compared with the industry’s decrease of 15.9%.
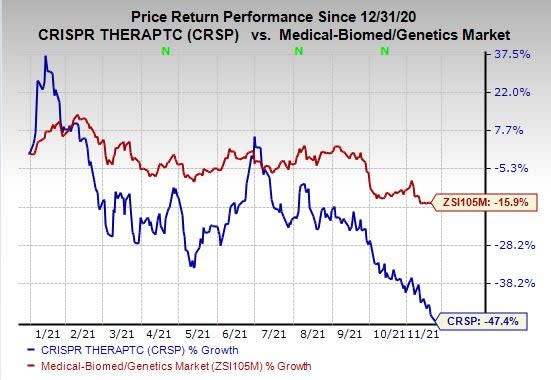
Image Source: Zacks Investment Research
We note that the phase I CARBON study is evaluating the safety and efficacy of several dose levels of CTX110 for treating relapsed/refractory B-cell malignancies. In October 2021, the company announced updated positive top-line data from the CARBON study that demonstrated the potential of CTX110 to produce durable remissions that are similar to the approved autologous CD19 CAR-T therapies on an intent-to-treat basis. Based on these results, the company plans to expand the CARBON study into a potential registrational study that is anticipated to begin consolidated dosing in first-quarter 2022.
CRISPR Therapeutics’ lead candidate is CTX001 — an investigational ex-vivo CRISPR gene-edited therapy it is developing for treating sickle cell disease and transfusion-dependent beta thalassemia — in partnership with Vertex Pharmaceuticals VRTX. The candidate is currently in development in a phase I/II study.
The company has achieved target enrollment in both the above studies and expects regulatory submission for the therapy in both indications by 2022-end.
Please note that CRISPR Therapeutics is solely dependent on Vertex for collaboration revenues. Hence, any termination of contract with Vertex will hurt the company’s growth prospects in the future.
Zacks Rank & Stocks to Consider
CRISPR Therapeutics currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector include Precision BioSciences, Inc. DTIL and Editas Medicine, Inc. EDIT, both sporting a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Precision BioSciences’ loss per share estimates have narrowed 44.4% for 2021 and 20.1% for 2022, over the past 60 days. The stock has rallied 10.1% year to date.
Earnings of Precision BioSciences have surpassed estimates in each of the trailing four quarters.
Editas Medicine’s loss per share estimates have narrowed 11.9% for 2021 and 4.1% for 2022, over the past 60 days.
Earnings of Editas Medicine have surpassed estimates in two of the trailing four quarters while missing the same on the other two occasions.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
Editas Medicine, Inc. (EDIT) : Free Stock Analysis Report
CRISPR Therapeutics AG (CRSP) : Free Stock Analysis Report
Precision BioSciences, Inc. (DTIL) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 