CureVac CEO talks Tesla tie-up and Q3 2021 COVID-19 vaccine approval

Germany’s BioNTech (BNTX) and its partner Pfizer (PFE) were the focus of global attention this week, after they announced their vaccine candidate showed 90% prevention-effectiveness based on preliminary Phase 3 trial data.
However, BioNtech is not the only German horse in the vaccine race. CureVac (CVAC), a biopharma company founded in 2002 in the south-eastern state of Baden-Würtemberg, is also going all-in to develop a vaccine against COVID-19.
WATCH: BioNTech founder says first UK patients could get jab next month
Like BioNTech, and Moderna (MRNA) and Translate Bio (TBIO) in the US, Curevac is also working with messenger RNA (mRNA) technology for its vaccine.
Unlike traditional vaccines, which involve putting weak or inactivated doses of a virus into the body to cause the immune system to produce antibodies, m-RNA vaccines work by transmitting a genetic code to cells telling them produce a protein, which in turn activates the immune system.
READ MORE: BioNTech founder on COVID-19 vaccine results: 'An important step for the world'
CureVac’s chief executive Franz-Werner Haas said in a video call with members of the foreign press, including Yahoo Finance UK, in Berlin today (12 November) that the company has had good feedback on its Phase 1 trials and is moving now into Phases 2 and 3, with recruitment of the 36,000 participants now underway.
“Rolling submissions” of their data and results to medical authorities will keep going on while the trials advance, and they are counting on final approval by the third quarter of 2021.
“A virus doesn’t differentiate between nationality, sex, skin colour, religion, it is an international problem, that must be tackled internationally,” Haas said on the call. “I will say it in the words of Bill Gates — he said it four years ago — such outbreaks will happen again and again. The thing is we don’t know what they will be and when they will come.”
Haas said today the innovative technology they are working on demands massive financial investment, pointing to the fact that Pfizer had put up $2bn at the start of this year in its cooperation with BioNTech.
CureVac, which went public on the Nasdaq in August, counts billionaire SAP (SAP) founder Dietmar Hopp, AstraZeneca (AZN.L), and the German government among its investors.
Berlin invested €300m and took a 23% stake in the company earlier this year. In September, the government said it would award €230m in grant funding to CureVac and €375m to BioNTech.
READ MORE: EU signs deal to buy 300 million doses of BioNTech-Pfizer COVID-19 vaccine
The company is in advanced talks with the European Commission to supply 225 million vaccine doses, with the option to buy another 180 million, and in talks with other parties. Haas declined to say who else they would be signing agreements with.
Even though there are number of huge pharmaceutical companies and their partners dashing to get a vaccine approved and sign supply deals all over the world, Haas does not envisage that the market will be totally saturated with different COVID-19 vaccines.
“We believe that there have to be a lot of vaccines at the beginning, because there won’t be enough production capacity, so there needs to be several players producing in parallel at the start,” he said.
“The other things is that one can assume that there will be different types of vaccines [needed], perhaps older people will need a different vaccine from younger people.”
Tesla on board

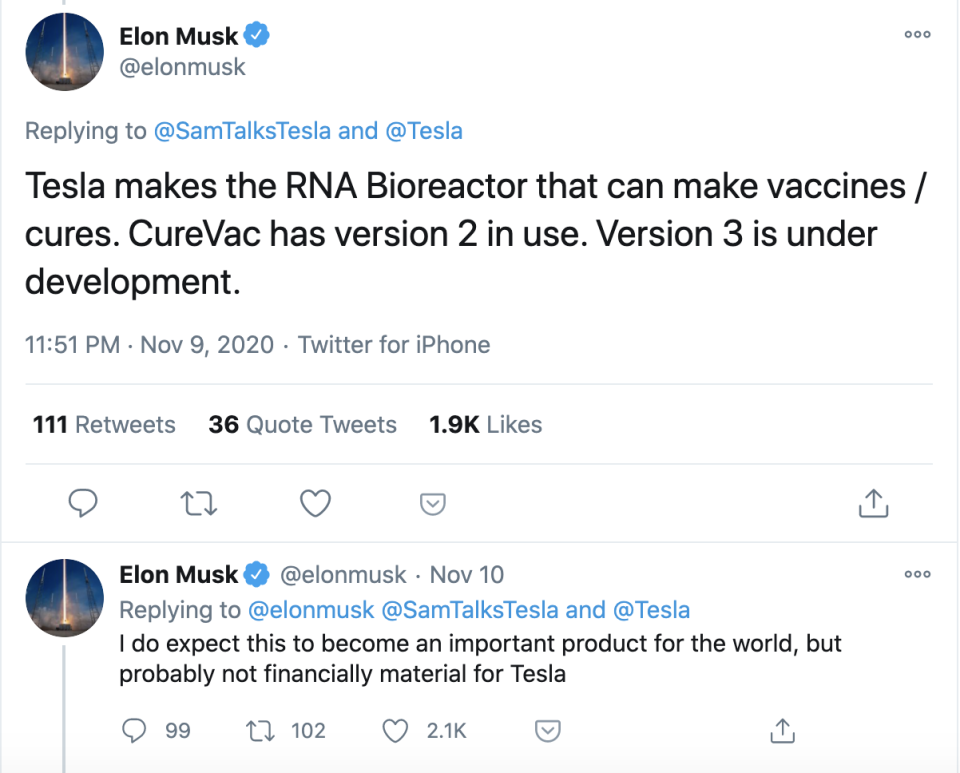
CureVac and Tesla’s German subsidiary Grohmann Automation have teamed up on the vaccine effort too, with Grohmann developing a special RNA reactor to produce vaccines in large quantities at speed.
“We also needed to think about how we must scale production,” Haas said today, noting that there are no traditional production facilities existing for this new type of mRNA technology. They believe it is important not only to mass produce huge amounts, but to be able to fulfil a need for smaller production capabilities.
That’s where Tesla Grohmann Automation came in. It is developing small RNA bioreactors, which can be set up in for example, a virus-hit area, and produce 100,000 doses a week. “That way you can really stop a virus from spreading,” Haas said. “It’s sort of like a cloud-solution... and we need these mobile solutions to shorten the logistics chain.”
“This is of course more expensive, because you don’t have the economies of scale, but we can produce it faster and more specifically… it is a sort of mobile production unit,” Haas said. “There we needed support — we are biologists and medics, and technicians, but we’re not machine builders.”
Tesla chief executive Elon Musk tweeted this week that Grohmann are already developing a third version for CureVac.

 Yahoo Finance
Yahoo Finance 