CVRx's (CVRX) Latest Data Favors Barostim's Long-Term Benefits
CVRx, Inc. CVRX recently announced the availability of additional data supporting the long-term benefits of Barostim. This includes the publication of results of the post-market phase of the BeAT-HF trial in the European Journal of Heart Failure. These data highlight the long-term sustained benefits of Barostim in heart failure (HF) patients with reduced ejection fraction.
It should be noted that BeAT-HF is a multi-center, prospective, randomized, controlled trial that began in April 2016 to develop scientific evidence for the safety and effectiveness of Baroreflex Activation Therapy with Barostim.
The manuscript is available online at the European Journal of Heart Failure website. CVRx had previously announced some of these data as part of expanded labeling granted by the FDA on Dec 23, 2023.
Also, two new post-hoc analyses of the BeAT-HF trial data, presented last month at Technology in Heart Failure Therapeutics (THT) 2024, suggest additional important benefits of Barostim.
The latest availability of additional data supporting the long-term benefits of Barostim is a major stepping stone for CVRx and is likely to solidify its position in the niche space.
Significance of the Data
Per management, the publication of these data from the post-market phase of BeAT-HF in the peer-reviewed journal is expected to allow for more effective dissemination of the long-term results of the important trial and the positive symptomatic impact of Barostim on patients with HF. Management was also upbeat about the favorable physician response to new abstracts released at THT last month that show a reduction in additional HF interventions in patients with Barostim, as well as specific patient-centered benefits at long-term follow-up.
Management is currently looking forward to the generation of additional evidence about Barostim from the BeAT-HF trial, as well as from real-world experience through CVRx’s REBALANCE post-market registry and investigator-initiated research.
Industry Prospects
Per CVRx, HF is one of the most prevalent cardiovascular diseases. The company estimates that there are approximately 26 million people globally suffering from HF, including approximately 6.2 million people in the United States and 8.6 million people in the European Five (Germany, France, Italy, Spain and the U.K.). Also, every year, many more new patients are diagnosed with HF in the United States and the European Five.
Management expects the company’s annual market opportunity for HF with reduced Ejection Fraction (HFrEF) to be $2.2 billion in the United States and $1.5 billion in select European Markets (the European Five).
Notable Development
In January, CVRx reported its fourth-quarter 2023 results, wherein it registered a solid uptick in its total revenues, revenue generated in the United States and U.S. HF revenues. The uptick in U.S. HF revenues was primarily driven by continued growth resulting from the expansion into new sales territories and new accounts and increased physician and patient awareness of Barostim.
Management confirmed that the company witnessed continued momentum in driving the adoption and utilization of Barostim, resulting in a 97% annual increase in U.S. HF revenues. It had also achieved significant milestones, such as the expansion of Barostim labeling and CMS' OPPS (hospital outpatient prospective system) ruling assigning Barostim to the New Technology payment code. Management believes these changes will enhance access to CVRx’s therapy.
Price Performance
Shares of CVRx have gained 61.9% in the past year against the industry’s 0.4% decline. The S&P 500 has witnessed 22.1% growth in the said time frame.
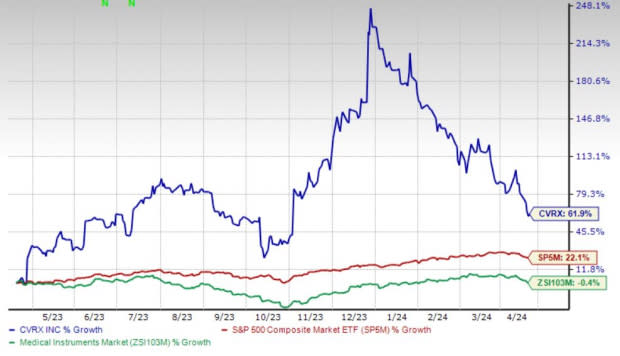
Image Source: Zacks Investment Research
Zacks Rank & Key Picks
Currently, CVRx carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the broader medical space are DaVita Inc. DVA, Ecolab Inc. ECL and Cencora, Inc. COR.
DaVita, carrying a Zacks Rank #2 (Buy) at present, has an estimated long-term growth rate of 12.1%. DVA’s earnings surpassed estimates in each of the trailing four quarters, with the average surprise being 35.6%. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
DaVita’s shares have gained 53.3% compared with the industry’s 9.6% rise in the past year.
Ecolab, carrying a Zacks Rank of 2 at present, has an estimated long-term growth rate of 13.3%. ECL’s earnings surpassed estimates in each of the trailing four quarters, with the average surprise being 1.7%.
Ecolab’s shares have rallied 33.4% against the industry’s 9.9% decline in the past year.
Cencora, carrying a Zacks Rank of 2 at present, has an estimated long-term growth rate of 9.8%. COR’s earnings surpassed estimates in each of the trailing four quarters, with the average surprise being 6.7%.
Cencora’s shares have rallied 43.2% compared with the industry’s 2.4% rise in the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Ecolab Inc. (ECL) : Free Stock Analysis Report
DaVita Inc. (DVA) : Free Stock Analysis Report
Cencora, Inc. (COR) : Free Stock Analysis Report
CVRx, Inc. (CVRX) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 