What we do and don’t know about the Pfizer Covid vaccine: Your questions answered

The UK has become the first country in the world to approve the Covid-19 vaccine developed by Pfizer and BioNTech, with the rollout of doses set to begin early next week.
Prime minister Boris Johnson welcomed the emergency authorisation, granted by the Medicines and Healthcare products Regulatory Agency (MHRA), and said it will help allow Britons to “reclaim our lives”.
Still, there are plenty of question marks surrounding the two-dose vaccine. So, what do and don’t we know?
When does vaccination start and how many doses will there be?
Matt Hancock, the health secretary, said the first 800,000 doses will be available in the UK as early as next week.
Up to 10 million doses were initially expected to be delivered before the end of the year, though Sean Marrett, BioNTech’s chief business officer, has said it is likely the UK will now receive half this figure in that time period.
Downing Street has ordered a total of 40 million doses – enough to vaccinate 20 million Britons.
Who will be vaccinated first?
It is still unclear who will be first to receive the new jab, despite the Joint Committee on Vaccination and Immunisation (JCVI) saying care home residents and staff should be prioritised.
The JCVI said these groups should be followed by those aged 80 and above and frontline health workers, then the clinically vulnerable.
However, the jabs are being initially delivered to NHS hospital hubs with sub-zero freezing capacity, to stop the vaccine vials from thawing too quickly. Mr Hancock said that 50 hospitals in UK are already set up and waiting to receive supplies.
People will be offered vaccination appointments – they shouldn't immediately call their GP requesting a jab.
Will care homes be able to receive doses?
While doses can physically be transported to care homes, further authorisation is needed from regulators before residents and staff can receive the coronavirus vaccine, Boris Johnson revealed at a Downing Street briefing on Wednesday evening.
The MHRA must provide further approval before the 975-dose boxes used to transport the vaccine supplies can be split up into batches suitable for distribution to homes, he said.
Care homes typically have dozens of residents: none have nearly a thousand. This means that if a batch is delivered to a care home then there would be a potential waste of precious vaccine stock.
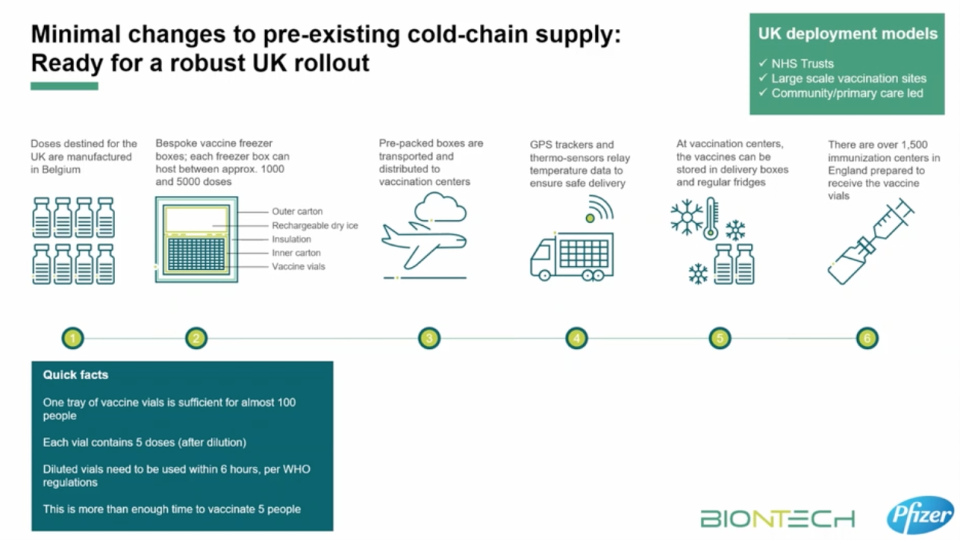
Doses can be transported from vaccination clinics or distribution sites as long as they travel for no more than six hours after leaving cold storage and are then placed in a normal fridge at 2C to 8C. The vaccine can survive at this temperature for five days.
Ben Osborn, Pfizer’s UK country manager, said: “The point I really want to emphasise is, at the point of administration and deployment by the NHS, our vaccine can be stored under normal refrigerated temperatures at 2-8C for five days.
“And that gives us the flexibility to reach the target populations identified this morning by the JCVI over the months ahead.”
It is understood that the vaccine can only be transported four times. It takes three moves to get the vials to storage hubs across Britain. So the final move will need to take place just before the vaccine is given to a patient.
Care providers said they were awaiting further details from the government on how the rollout will start, who will be administering the vaccinations and where it will be done.
With so many hurdles to clear, Mr Johnson has warned that “it will inevitably take some months before all the most vulnerable are protected”.
Has the MHRA moved too quickly in granting approval?
There has been some criticism of the speed at which the MHRA moved in assessing the Pfizer-BioNTech vaccine.
European lawmakers described the UK’s emergency market authorisation of the Pfizer/BioNTech vaccine as “problematic”, while the EU’s drug regulator – the European Medicines Agency – urged members states not to grant “hasty” approvals.
The request for approval was first submitted by BioNTech and Pfizer to the MHRA last month, though the agency – along with other regulators across the world – had already begun a rolling review of the phase-three trial data in October to accelerate the approval process.
This allowed the MHRA to start putting together a picture of the vaccine’s safety and efficacy as the data arrived.
The European Commission, the EU executive, said the EMA's authorisation procedure was “the most effective regulatory mechanism to grant all EU citizens' access to a safe and effective vaccine”, as it was based on more evidence and required more checks than the MHRA’s review.
However, the MHRA insisted “no corners have been cut”. Dr June Raine, head of the UK regulator, said the recommendation followed “the most rigorous scientific assessment of every piece of data so that it meets the required strict standards of safety, of effectiveness and of quality”.
The checks conducted by the MHRA are “equivalent to all international standards”, she added.
Pfizer and BioNTech echoed such statements. Ozlem Tureci, chief medical officer at BioNTech, said the company has had “very positive experiences with all regulators around the world, including MHRA, FDA [the US Food and Drug Administration] and EMA.”
Mr Marrett said: “The MHRA has asked the same level of detailed questions as any other agency and have focused on efficacy, the tolerance of the drug and the quality of production”. He added: “These three elements are key elements to any vaccine that any regulator will look in detail at. I think the MHRA has been no different in that respect.”
Explaining the delay between the MHRA and EMA – which intends to provide a decision on approval by 29 December – Mr Osborn said: “We have provided complete data packages, the unblinded data, to both regulators. I think what you're seeing is just the difference in the underlying process and timelines, as opposed to any difference in data submission.”
Has Brexit played a role in the quick approval?
There have been some suggestions from politicians that Brexit enabled the UK to move quicker in approving the vaccine compared to its European counterparts. This isn’t true.
While vaccines would typically be authorised by the EMA – at least, until the end of the Brexit transition on 31 December – certain member states of the EU, including the UK, have the power to temporarily authorise medical products in times of urgent public need.
Downing Street declined to be drawn on claims by Mr Hancock that the UK was able to approve the Pfizer vaccine more quickly because of Brexit.
According to Peter Liese, an EU lawmaker who is a member of German chancellor Angela Merkel’s party, the authorisation is “not a Brexit issue”. Germany could also have approved the vaccine under emergency powers, he explained, but EU member states had decided to wait for the green light from the EMA.
"The other member states think that it is necessary to look more carefully.”
And Dr Raine also contradicted Mr Hancock’s claims, and said the process was undertaken under the terms of European law, which remains in force until the completion of the Brexit transition period at the end of 2020.
How will the vaccine be kept cold?
Once the vaccine supply arrives in the UK, it will undergo quality checks to ensure it has been shipped safely.
Doses can either be stored in “freezer farms” at -70C, which will maintain the integrity of the doses for up to six months, or transported to mass vaccination clinics and distribution sites.
To get around the problem of the cold chain, Pfizer has developed a specially built deep-freeze “suitcase” that can be tightly sealed and transported even in non-refrigerated trucks.
These boxes are with packed with dry ice and installed with GPS trackers, which will allow Pfizer to remotely track the location and temperature of the frozen vaccine vials. Each reusable box can keep up to 5,000 doses of the vaccine at the right temperature for 10 days.
These will allow authorities to move doses to the UK’s dedicated vaccination centres. A number of venues have already been earmarked, including sports halls, leisure centres and even the Copper Box stadium in London’s Olympic Park.
Once delivered, the vaccine can be stored in traditional fridges (2C-8C) for up to five days, meaning authorities will have to move quickly in administering doses among priority groups.
So, when will life return to normal?
Mr Hancock conceded he is unsure how many Britons will need to receive a Covid-19 vaccine before restrictions can be lifted.
The health secretary told MPs that while the vaccine has been shown to offer protection, it is unclear what impact it has on reducing the transmission of Covid-19. Similarly, there is no indication yet as to how long immunity lasts for.
It is hoped rates of the virus will decrease as more vulnerable people are vaccinated thereby paving the way to lift restrictions, Mr Hancock explained.
Others have been more optimistic. “I wouldn’t be surprised if we hit the new year with two or three vaccines, all of which could be distributed,” Sir John Bell, a member of the government’s Scientific Advisory Group for Emergencies (Sage), said last month.
“That's why I’m quite optimistic of getting enough vaccinations done in the first quarter of next year that by spring things will start to look much more normal than they do now.”
Read More
Not enough Covid vaccine stock to prevent imminent surge in cases: WHO
One in five reluctant to get Covid vaccine, survey suggests
Vaccine rollout ‘biggest logistical effort nation has faced’ - Hancock
What we know about the safety of Covid Pfizer vaccine

 Yahoo Finance
Yahoo Finance 