Editas (EDIT) Gets FDA Nod to Begin Safety Study on EDIT-301
Editas Medicine, Inc. EDIT announced that the FDA has cleared the initiation of the safety phase of its clinical study on investigational ex vivo gene-editing therapy EDIT-301 being developed for the treatment of sickle cell disease (“SCD”). The company is planning to initiate the phase I/II RUBY study to assess the safety and efficacy of EDIT-301 for the given indication.
SCD is caused by a mutation in the beta-globin gene that causes polymerization of the sickle hemoglobin protein (HbS).
Per the press release, Editas has identified a lead principal investigator and engaged a Clinical Research Organization. Meanwhile, before starting enrollment for the efficacy phase of the RUBY study, Editas will need to resolve a partial clinical hold by developing an improved potency assay and submiting the same to the FDA.
EDIT-301 is an experimental autologous cell therapy medicine under investigation for treating SCD. The FDA has already granted a Rare Pediatric Disease designation to EDIT-301. The candidate is being developed as a best-in-class one-time durable medicine for people suffering from SCD.
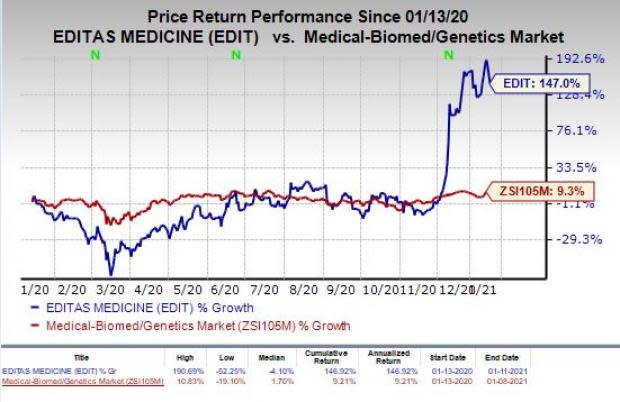
Shares of Editas were down 15.1% on Monday despite the encouraging news. However, the stock has skyrocketed 147% in the past year compared with the industry’s increase of 9.3%.
Editas currently has no approved product in its portfolio. Therefore, its pipeline development remains its key focus.
We note that EDIT-301 is Editas’ second pipeline candidate to enter clinical testing. The company’s lead candidate is EDIT-101, which employs CRISPR gene editing to treat LCA10 — a rare genetic illness that causes blindness. Editas completed dosing in the first cohort of the phase I/II BRILLIANCE study, which is evaluating EDIT-101 for LCA10.
LCA10 disease has a significant unmet need as no therapy is presently approved to cure the disease either in the United States or in the European Union.
Notably, apart from Editas, other companies are also using the CRISPR/Cas9 gene editing technology to develop their respective candidates for addressing various ailments. Intellia Therapeutics NTLA and CRISPR Therapeutics CRSP are among a few companies engaged in developing candidates to address different indications using CRISPR/Cas9 gene-editing technology.
Zacks Rank & Stock to Consider
Editas currently carries a Zacks Rank #4 (Sell).
A top-ranked stock in the biotech sector is Halozyme Therapeutics, Inc. HALO, which has a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Halozyme’s earnings estimates have been revised 3.6% upward for 2021 over the past 60 days. The stock has soared 127.8% in the past year.
Looking for Stocks with Skyrocketing Upside?
Zacks has just released a Special Report on the booming investment opportunities of legal marijuana.
Ignited by referendums and legislation, this industry is expected to blast from an already robust $17.7 billion in 2019 to a staggering $73.6 billion by 2027. Early investors stand to make a killing, but you have to be ready to act and know just where to look.
See the pot stocks we're targeting >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Halozyme Therapeutics, Inc. (HALO) : Free Stock Analysis Report
Editas Medicine, Inc. (EDIT) : Free Stock Analysis Report
Intellia Therapeutics, Inc. (NTLA) : Free Stock Analysis Report
CRISPR Therapeutics AG (CRSP) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 