Exelixis (EXEL) Announces Preliminary Q4 Results, Issues View (Revised)
Exelixis, Inc. EXEL recently announced its preliminary results for the fourth quarter and full-year 2021 and provided financial guidance for full-year 2022.
In 2021, cabozantinib generated approximately $1.08 billion in preliminary net product revenues in the United States.
Lead drug, Cabometyx (cabozantinib tablets), which is approved for advanced renal cell carcinoma (RCC) and previously treated hepatocellular carcinoma (HCC), maintains momentum on label expansions.
For the fourth quarter, the company has generated net product revenues of approximately $300 million.
For 2022, the company expects net product revenues of $1.325 billion to $1.425 billion.
In January 2021, the FDA approved Cabometyx in combination with immuno-oncology drug Bristol-Myers’ BMY Opdivo for the first-line treatment of patients with advanced RCC. Sales of the drug saw an increase in volume in the second quarter, driven by the strong uptake for the combination therapy of Cabometyx and Opdivo.
Sales of the drug saw an increase in volume, driven by the strong uptake for the combination therapy of Cabometyx and Opdivo.
Opdivo is one of the main growth drivers for Bristol Myers’. The company is working on expanding the drug’s label in various other indications through combination studies with other drugs.
Exelixis is looking to build a differentiated next-generation pipeline in oncology through strategic collaborations. The successful development of additional candidates will diversify its revenue base and reduce dependence on lead drug Cabometyx.
Exelixis’ shares have lost 22.4% in the past year compared with the industry’s decline of 31%.
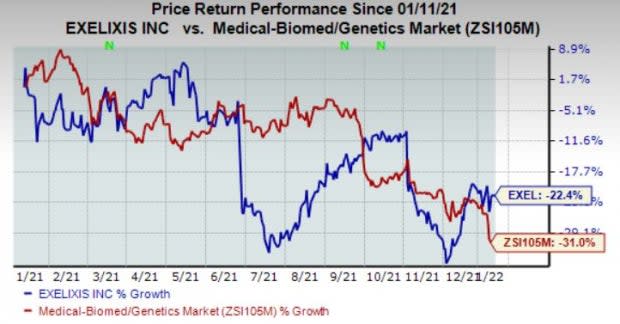
Image Source: Zacks Investment Research
The company also outlined corporate priorities for 2022. It expects top-line results from COSMIC-313, the phase III study evaluating the triplet combination of cabozantinib, Opdivo and Yervoy versus the combination of Opdivo and Yervoy in patients with previously untreated advanced intermediate- or poor-risk RCC. It also expects final overall survival data from COSMIC-312, the phase III study evaluating the combination of cabozantinib and Tecentriq in previously untreated HCC; and initial data in the second half of 2022 from CONTACT-01 and CONTACT-03, the phase III studies that are evaluating cabozantinib in combination with Tecentriq for non-small cell lung cancer (NSCLC) and RCC, respectively.
Exelixis expects to initiate the first global phase III study for XL092 in the first half of 2022. The first trial, STELLAR-303, will evaluate XL092 in combination with Tecentriq versus regorafenib in patients with metastatic microsatellite stable colorectal cancer (CRC) who have progressed after or are intolerant to the current standard of care.
Exelixis expects to expand the ongoing phase Ib STELLAR-001 and STELLAR-002 studies, which are evaluating XL092 in combination with several IO therapies, into potential new tumor types, and IO and other targeted therapy combination regimens throughout 2022. Updates from these trials are expected in 2022.
The single-agent dose-escalation cohort for the ongoing phase I study of XB002 continues enrolling, and the study is expected to move into its cohort expansion and combination phase shortly.
The ongoing phase I study of XL102, a potent, selective and orally bioavailable small molecule cyclin-dependent kinase 7 (CDK7) inhibitor, is currently in its dose-escalation phase, with enrollment ongoing in the single-agent and combination therapy cohorts.
Exelixis expects to initiate dosing in the phase I study of XL114 in patients with non-Hodgkin’s lymphoma during the first half of 2022.
However, competition is stiff in the RCC market. Hence, Exelixis is looking to develop its portfolio beyond Cabometyx.
Merck’s MRK Keytruda in combination with Pfizer’s PFE Inlyta is also indicated for the first-line treatment of patients with advanced RCC.
Merck’s Keytruda, an anti-PD-1 therapy, is approved for the adjuvant treatment of patients with RCC at intermediate-high or high risk of recurrence, following nephrectomy or nephrectomy and resection of metastatic lesions.
Pfizer’s Inlyta has shown strong performance, driven by continued adoption in the United States and Europe. Pfizer’s older drug Sutent is also approved for advanced RCC.
Exelixis currently carries a Zacks Rank #4 (Sell).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
(We are reissuing this article to correct a mistake. The original article, issued on January 10, 2022, should no longer be relied upon.)
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Exelixis, Inc. (EXEL) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 