FATE Reports Wider-Than-Expected Q2 Loss, Beats on Revenues
Fate Therapeutics FATE reported a loss of 58 cents per share in the second quarter of 2021, wider than the year-ago loss of 35 cents and the Zacks Consensus Estimate of a loss of 52 cents.
Increased research & development (R&D) and general & administrative (G&A) expenses led to the wider loss.
The company earned collaboration revenues of $13.4 million in the second quarter, which easily beat the Zacks Consensus Estimate of $7 million, and were up from $5.4 million reported in the year-ago quarter. Revenues are primarily derived from the company’s collaborations with Janssen, a unit of Johnson & Johnson JNJ, and Ono Pharmaceutical.
R&D expenses surged to $48 million from $26.7 million in the year-ago quarter.
G&A expenses jumped to $12.2 million from $7.5 million in the year-ago quarter.
Cash, cash equivalents and investments at the end of the second quarter were $845.1 million.
Pipeline Update
The dose-escalating phase I study of FT596 for patients with relapsed / refractory B-cell lymphoma (BCL) has successfully cleared dose-limiting toxicity in dose Cohort 3 (single dose of 300 million cells) as monotherapy and in combination with Rituxan. Dose escalation of the single-dose treatment schedule is ongoing in both regimens with enrollment in dose Cohort 4 (900 million cells). Fate is also planning to initiate enrollment of a multi-dose treatment schedule in both regimens, with FT596 administered on day 1 and day 15 at 300 million cells / dose with the potential to dose escalate to 900 million cells / dose.
In July, the first patient was treated in the phase I study of FT819, the first-ever T-cell therapy derived from a clonal master induced pluripotent stem cell (iPSC) line to undergo clinical investigation. FT819 is an off-the-shelf, allogeneic CAR T-cell therapy targeting CD19 and the first patient received a single FT819 dose of 90 million cells for the treatment of relapsed / refractory acute lymphoblastic leukemia (ALL).
In June, Fate and Janssen elected to initiate IND-enabling activities for an iPSC-derived CAR NK cell product candidate incorporating a Janssen proprietary antigen binding domain that targets an antigen expressed on certain solid tumors. This move triggered the payment of a milestone fee to the company by Janssen under the collaboration.
Fate plans to submit an IND application to the FDA in the second half of 2021 to initiate a phase I study of FT536 for the treatment of solid tumors.
The company also plans to initiate enrollment at 100 million cells per dose upon clearance of the first dose cohort in its Phase 1 study of FT538 in relapsed / refractory AML.
Our Take
Higher R&D expenses hit the bottom line in the second quarter. Nevertheless, cellular immunotherapies promise huge potential and hence, the successful development of its product candidates will be a significant boost for the company.
Companies like Gilead Sciences, Inc. GILD and Bristol-Myers Squibb Company BMY are currently focusing on developing cellular immunotherapies to treat cancer.
Shares of Fate have lost 5.7% in the year so far against the industry’s growth of 1%.
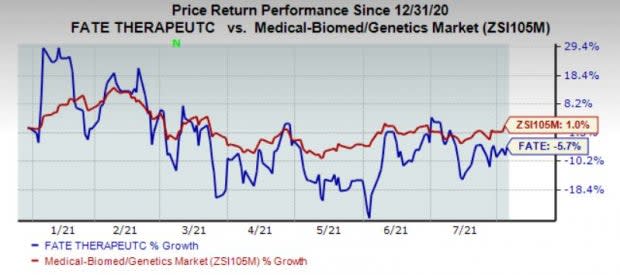
Image Source: Zacks Investment Research
Fate currently carries a Zacks Rank #4 (Sell).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
Fate Therapeutics, Inc. (FATE) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 