FDA Limits Use of Regeneron & Lilly's COVID Antibody Cocktails
The FDA announced that it has revised the emergency use authorization (EUA) for two cocktail antibody drugs — Eli Lilly’s LLY bamlanivimab plus etesevimab, and Regeneron Pharmaceuticals’ REGN REGEN-COV (casirivimab plus imdevimab). The revision for these two cocktail COVID-19 drugs limits their use in patients who have been infected or exposed to a coronavirus variant that is susceptible to these treatments.
The FDA stated that Eli Lilly and Regeneron’s COVID-19 drugs are currently not authorized for use in any U.S. states, territories, and jurisdictions. However, the FDA mentioned that these drugs may be authorized for use in certain regions where there is a chance of COVID-19 infections due to coronavirus variants susceptible to these treatments.
The FDA’s decision to limit the authorized use of these cocktail COVID-19 drugs was based on data, which showed that these treatment options are highly unlikely to be effective against the currently prevalent Omicron variant in the United States. Data as of Jan 15 from the Centers for Disease Control and Prevention have shown that more than 99% of COVID-19 infection cases in the United States are estimated to be due to the Omicron variant.
The COVID-19 Treatment Guidelines Panel of the National Institute of Health (“NIH”) also recently recommended against the use of Eli Lilly and Regeneron’s COVID-19 drugs due to their markedly reduced activity against the Omicron variant. Moreover, the NIH panel stated that real-time testing for identifying rare non-Omicron variants is not available routinely, which poses a challenge for the effective use of these drugs in fighting COVID-19.
We note that Regeneron’s biologics license application seeking “full” approval for REGEN-COV as treatment in non-hospitalized COVID-19 patients is under review with the FDA. A decision is expected in April. However, the FDA is unlikely to grant approval for the drug following limiting its EUA.
While Lilly’s bamlanivimab and bamlanivimab/etesevimab cocktail medicines generated revenues of $217.1 million in the third quarter of 2021, Regeneron generated $1.2 billion from the sale of REGEN-COV. The majority of revenues from these drugs were generated in the United States. The limitation in the authorized use of these drugs should hurt the top line of Lilly and Regeneron beginning the first quarter of 2022.
While shares of Lilly declined 1.09% on Jan 24 following the FDA update, Regeneron’s shares were down 1.06% in after-hours trading on the same day.
Shares of Lilly have gained 13.2% in the past year compared with the industry’s increase of 9.7%.
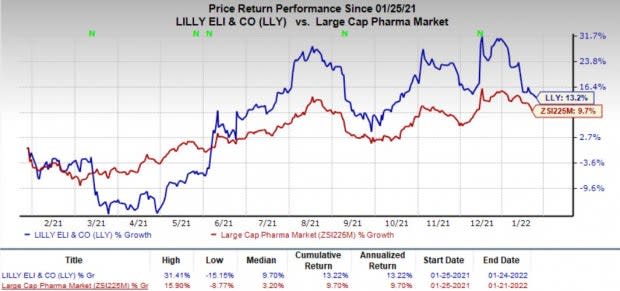
Image Source: Zacks Investment Research
Over the past 12 months, Regeneron’s shares were up 13.4% against the industry’s decrease of 40.7%.
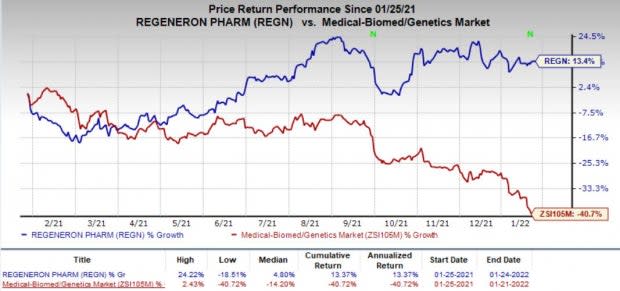
Image Source: Zacks Investment Research
The FDA has always vouched for the best tools to fight against the COVID-19 pandemic since its beginning. The FDA stated that several other options are available to treat COVID-19 infections and they are expected to be effective against the currently prevalent Omicron variant. These treatment options include oral drugs —Pfizer’s PFE Paxlovid and Merck’s MRK molnupiravir — and intravenous drugs — Glaxo’s sotrovimab and Gilead’s Veklury.
Pfizer’s Paxlovid and Merck’s molnupiravir are the first oral treatment options in the non-hospitalized setting for COVID-19 infection that were authorized for emergency use in December 2021 in the United States. While Pfizer expects to manufacture 120 million courses of Paxlovid by the end of 2022, Merck expects molnupiravir’s global opportunity to be approximately $5 billion to $7 billion through 2022.
The unavailability of two popular COVID-19 drugs may boost the demand for the existing COVID-19 treatment options, especially the oral drugs as they are easy to administer.
While Pfizer sports a Zacks Rank #1 (Strong Buy), Regeneron carries a Zacks Rank #2 (Buy). Merck and Lilly carry a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 