Glaxo (GSK), SK bioscience's COVID-19 Jab Enters Phase III Study
GlaxoSmithKline plc GSK, along with Korean biopharmaceutical company SK bioscience, announced that they have initiated a phase III study on the latter’s nanoparticle COVID-19 vaccine candidate, GBP510, which is being developed using Glaxo’s pandemic adjuvant.
The randomized phase III study will evaluate the safety and immunogenicity of GBP510 as compared to an active comparator – the Oxford University and AstraZeneca’s AZN COVID-19 vaccine. The study will enroll approximately 4,000 participants from various countries, and is likely to be one of the first phase III studies to compare different COVID-19 vaccine candidates.
Data from the above-mentioned study is expected in the first half of 2022. If successfully developed and upon potential approval, the vaccine is expected to be supplied at scale through the COVAX facility across the globe.
Glaxo and SK bioscience decided to advance the vaccine candidate to the phase III study after interim data from the phase I/II study showed that all participants who received the adjuvanted vaccine candidate developed strong neutralizing antibody responses with a 100% seroconversion rate.
Shares of Glaxo have increased 10.7% so far this year compared with the industry’s growth of 16.2%.
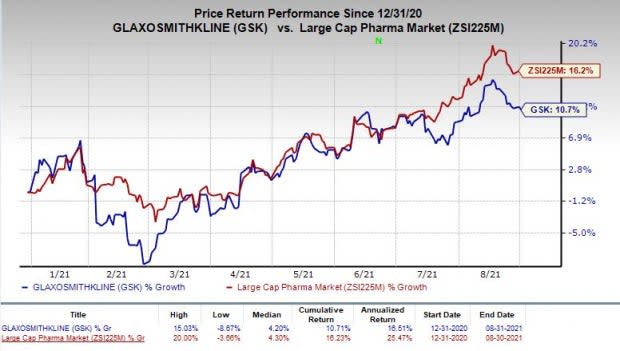
Image Source: Zacks Investment Research
We note that Glaxo entered into multiple partnerships with various companies like Sanofi SNY, Medicago, SK Biosciences and others to help make their COVID-19 vaccine candidates in combination with Glaxo’s pandemic adjuvant. Sanofi’s adjuvanted COVID-19 vaccine candidate is currently in phase III development. Glaxo also has a partnership with German biopharmaceutical company CureVac N.V. CVAC to develop the next-generation mRNA COVID vaccines.
In the second quarter of 2021, Glaxo recorded sales of £260 million from its pandemic vaccines, including £258 million of pandemic adjuvant sales. Potential approval for COVID-19 vaccines should boost sales further in future quarter.
Other than vaccines, Glaxo is also making therapies for COVID-19. In May 2021, the FDA granted an emergency use authorization to Glaxo and Vir Biotechnology, Inc.’s sotrovimab, an investigational single-dose monoclonal antibody, for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients. Sotrovimab is currently under review in the EU.
Zacks Rank
Glaxo currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
CureVac N.V. (CVAC) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 