Global Radioligand Therapy Markets, 2020-2021 & 2022-2031: Expanding Radiopharmaceutical Coverage & Radioligand Therapy Demand Inclined by Rising Prevalence of Cancer

Global Radioligand Therapy Market
Dublin, May 11, 2022 (GLOBE NEWSWIRE) -- The "Radioligand Therapy Market - A Global and Regional Analysis: Focus on Indication, Product, Biomarker, and Region - Analysis and Forecast, 2021-2031" report has been added to ResearchAndMarkets.com's offering.
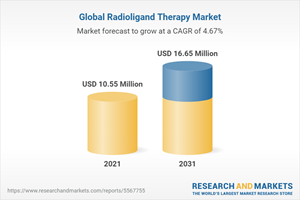
The global radioligand therapy market was valued at $9,754.9 million in 2020 and is projected to reach $16,658.4 million by 2031, reveals the premium market intelligence study by the publisher. The study also highlights that the market is set to witness a CAGR of 4.67% during the forecast period 2021-2031.
The study encompasses the market growth drivers, market restraining factors, opportunities, competition mapping, and segmental analysis.
This study indicates that the growing prevalence of cancer, strategic initiatives by key market players, and rise in microbial sequencing are the major factors anticipated to contribute to the growth of the global radioligand therapy market.
Data from different segments of the market has been analyzed minutely to gain a holistic view of the market. These segments include market segmented based on indication, product, biomarker, and region. Each of these segments is further categorized into sub-segments and micro-segments to compile an in-depth study.
Key insights are drawn from in-depth interviews with the key opinion leaders of 16 leading companies, market participants, and vendors.
In the comprehensive study of the global radioligand therapy market, the publisher extensively covers the following:
Market numbers on micro-segments influencing the market
Market share analysis of key market players
Growth share analysis of companies
Impact of COVID-19 on the global radioligand therapy market
Growth analysis by indication, product, biomarker, and region
Detailed company and product profiling for 16 companies
Pipeline analysis for potential pipeline drugs
The study highlights the following in the report:
Emerging opportunities in the radioligand therapy market
Impact of COVID-19 on the global radioligand therapy market
Market scenario for radioligand therapy marketed products and potential pipeline products
Drivers promoting the growth of the market
Insightful Questions Covered to Enable Companies Take Strategic Decisions
How is radioligand therapy revolutionizing oncology?
What are the major market drivers, challenges, and opportunities in the global radioligand therapy market?
What are the underlying structures resulting in the emerging trends within the global radioligand therapy market?
How is the COVID-19 pandemic impacting the global radioligand therapy ecosystem?
What are the key development strategies that the major players are implementing in order to sustain themselves in the competitive market?
What are the key regulatory implications in developed and developing regions pertaining to the use of radioligand-targeted therapies?
What are the potential entry barriers expected to be faced by the companies willing to enter a particular region?
How is each market segment expected to grow during the forecast period 2021-2031, and what is the anticipated revenue to be generated by each segment? Following are the segments:
Products (Approved Products and Potential Pipeline)
Indication (Prostate Cancer, Neuroendocrine Tumor (NETs), and Others)
Biomarker (Prostate-Specific Membrane Antigen, Ki 67 Expression and Grading, Cytochrome P450 17A1 Inhibitor)
Region (North America, Europe, Asia-Pacific, and Rest-of-the-World)
What are the growth opportunities for the radioligand therapy companies in the region of their operation?
Who are the leading players with significant offerings in the global radioligand therapy market?
Which companies are anticipated to be highly disruptive in the future, and why?
Key Topics Covered:
1 Research Methodology
1.1 Radioligand Therapy: Research Methodology
1.2 Primary Data Sources
1.3 Secondary Data Sources
1.4 Market Estimation Model
1.5 Criteria for Company Profiling
2 Radioligand Therapy Market
2.1 Product Definition
2.1.1 Inclusion and Exclusion
2.2 Market Scope
2.2.1 Scope of the Study
2.2.2 Key Questions Answered in the Report
2.3 Market Overview
2.3.1 Market Definition
2.3.2 Emerging Applications of Radioligand Therapy
2.3.2.1 Neuroendocrine Neoplasms
2.3.2.2 Prostate Cancer
2.3.2.3 Non-Cancerous Condition
2.3.3 Market Footprint and Growth Potential
2.3.4 COVID-19 Impact on Global Radioligand Therapy Market
2.3.4.1 Impact on Radioligand Therapy
2.3.4.2 Clinical Trial Disruptions and Resumptions
3 Industry Analysis
3.1 Overview
3.2 Challenges in Radioligand Therapy Regulatory Approval Pathway
3.3 Regulatory Scenario of Radioligand Therapy
3.4 Legal Requirements and Frameworks in the U.S.
3.4.1 Clinical Trial Authorization
3.4.2 Marketing Authorization
3.4.3 U.S. FDA Guidelines for NDA Submission
3.4.4 Post-Authorization Regulations
3.5 Legal Requirements and Frameworks in Europe
3.5.1 EMA Drug License Application Process
3.5.2 Centralized Procedure
3.5.3 Decentralized Procedure
3.5.4 Mutual-Recognition Procedure
3.5.5 National Procedure
3.6 Legal Requirements and Frameworks in Asia-Pacific
3.6.1 Legal Requirements and Frameworks in Japan
4 Pipeline Analysis
4.1 Radioligand Therapy Pipeline Analysis
4.2 Radioligand Therapy Clinical Trial Design
4.2.1 [Lu-177]-PNT2002
4.2.1.1 Product Profile
4.2.1.2 [Lu-177]-PNT2002 Phase III Study Design
4.2.1.3 [Lu-177]-PNT2002 Efficacy, Safety, and Tolerability (Phase II)
4.2.1.4 177Lu-PNT2002 Pharmacokinetics and Pharmacodynamics Profile (Phase I)
4.2.2 CAM H2
4.2.3 [177Lu]-NeoB
4.2.4 PNT-2003
4.2.5 ITM-11
4.2.6 177.Lu FAP-2286
4.2.7 FPI-1434
4.2.8 FPI-1966
4.2.9 177Lu-DOTA-rosopatamab
5 Market Dynamics
5.1 Overview
5.2 Impact Analysis
5.3 Market Drivers
5.3.1 Radioligand Therapy Demand Inclined by Rising Prevalence of Cancer
5.3.2 Strategic Initiatives by Key Market Players
5.3.3 Rise in Clinical Research Activity
5.4 Market Restraints
5.4.1 High Cost Associated with Treatment and Complex Reimbursement Processes
5.4.2 Increased Competition from Generics
5.5 Market Opportunities
5.5.1 Expanding Radiopharmaceutical Coverage
5.5.2 Role of Radioligand in Drug Discovery
6 Competitive Landscape
6.1 Overview
6.2 Product Launch
6.3 Product Approvals
6.4 Synergistic Activities
6.5 Mergers and Acquisitions
6.6 Market Share Analysis (by Company), 2020
7 Global Radioligand Therapy Market, Indication, $Million, 2020-2031
7.1 Overview
7.2 Prostate Cancer
7.3 Neuroendocrine Tumor (NETs)
7.4 Other
8 Global Radioligand Therapy Market, Products, $Million, 2020-2031
8.1 Overview
8.2 Approved Products
8.2.1 Lutathera
8.2.2 Zytiga
8.2.3 Xtandi
8.2.4 Xofigo
8.3 Potential Pipeline
9 Global Radioligand Therapy Market, Biomarker, $Million, 2020-2031
9.1 Overview
9.2 Prostate-Specific Membrane Antigen
9.3 Ki 67 Expression and Grading
9.4 Cytochrome P450 17A1 Inhibitor
10 Global Radioligand Therapy Market, Region, $Million, 2020-2031
10.1 Overview
11 Markets - Competitive Benchmarking & Company Profiles
11.1 Overview
11.2 Company Overview
11.3 Role of in the Global Radioligand Therapy Market
11.4 Product ASP (by Region)
11.5 Key Competitors of Company
11.6 Financials
11.7 Key Insights About the Financial Health of the Company
Johnson & Johnson Services, Inc.
Pfizer Inc.
Amneal Pharmaceuticals LLC
Novartis International AG
POINT Biopharma Global Inc
Fusion Pharma
Clovis Oncology
Telix Pharmaceuticals
Lantheus Holdings, Inc. (Progenics Pharmaceuticals)
Bayer AG
Molecular Partners
ITM Isotope Technologies Munich SE
Curium Pharma
Precirix
Radio Medix
For more information about this report visit https://www.researchandmarkets.com/r/bywjom
Attachment
CONTACT: CONTACT: ResearchAndMarkets.com Laura Wood, Senior Press Manager press@researchandmarkets.com For E.S.T Office Hours Call 1-917-300-0470 For U.S./CAN Toll Free Call 1-800-526-8630 For GMT Office Hours Call +353-1-416-8900


 Yahoo Finance
Yahoo Finance 
