GSK & Others Get Relief as US Court Dismisses Zantac Litigation
GSK GSK issued a statement welcoming the decision of a Multi-District Litigation (MDL) court of Southern Florida, which dismissed all lawsuits alleging that the heartburn drug Zantac (ranitidine) causes cancer.
In the MDL, plaintiffs identified five different types of cancers (liver, bladder, pancreatic, esophageal and stomach). There were around 50,000 claims in the MDL. The court dismissed all MDL cases alleging the five cancers.
Other than GSK, the U.S. district court verdict brought relief to other companies, which marketed prescription or over-the-counter (OTC) Zantac (ranitidine) medicines such as Sanofi SNY and Pfizer PFE. If the claims in the MDL had been proved, the liabilities of these drugmakers could have been in billions.
GSK’s stock has declined 33.1% this year so far compared with a decline of 18.7% for the industry.
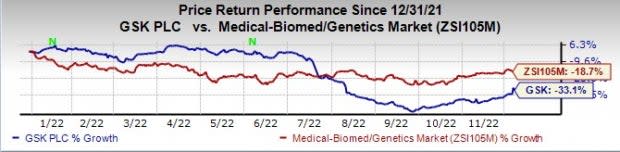
Image Source: Zacks Investment Research
In April 2020, the FDA requested manufacturers to withdraw all prescription and OTC Zantac (ranitidine) medicines from the market with immediate effect. The regulatory agency decided to take this step due to a contaminant known as N-Nitrosodimethylamine (NDMA) found in ranitidine drugs.
NDMA is a substance that can cause cancer in humans. In 2019, the FDA became aware of the low levels of NDMA traces found in ranitidine in laboratory testing. Subsequently, the regulatory body decided to caution the general public against taking ranitidine drugs and, instead, consider alternative treatments.
Eventually, the companies and manufacturers decided to withdraw and recall all the ranitidine products from the market.
Several personal injury cases were filed in federal and state courts, alleging that Zantac (ranitidine) medicines cause cancer. However, GSK, Pfizer and Sanofi denied that Zantac causes cancer, citing scientific consensus.
The statement released by GSK mentioned that there was no consistent or reliable evidence to show that ranitidine increases the risk of any cancer and hence the lawsuits were dismissed.
Though several thousands of cases remain pending in various U.S. state courts and the MDL ruling can still be appealed, it meaningfully reduces the companies’ liabilities and removes a major overhang.
Zacks Rank
GSK currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
GSK PLC Sponsored ADR (GSK) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 