Inari Medical (NARI) Launches New Catheters to Address VTE
Inari Medical, Inc. NARI recently announced the commercial launch of two new purpose-built products, the RevCore thrombectomy catheter and the Triever16 Curve catheter. RevCore is currently the first mechanical thrombectomy device designed to address venous in-stent thrombosis, whereas the Triever16 Curve catheter is the latest addition to Inari Medical’s FlowTriever platform.
The latest launches are expected to significantly solidify Inari Medical’s foothold in the Venous Stent Thrombosis and Venous Thromboembolism (VTE) treatment space globally.
Significance of the Launch
Per Inari Medical’s estimates, approximately half a million venous stents have been placed in the United States in the past few years. However, the company also confirmed that venous in-stent thrombosis is an increasingly common occurrence, affecting more patients every year. Inari Medical believes that RevCore will likely be able to address this issue as this is a solution for an entirely new patient pool, which is not currently addressed by the ClotTriever or FlowTriever platforms.
Per Inari Medical, the Triever16 Curve catheter, which features a pre-shaped curve for targeted aspiration, is purpose-built to be versatile for both pulmonary embolism and peripheral thrombectomy. Triever16 Curve offers unique advantages over 16F continuous aspiration catheters, including compatibility with the FlowSaver blood return system and simple access to larger, more powerful 20F or 24F catheters within the company’s price-per-procedure model.
An expert familiar with RevCore believes that it is an innovative tool as it physically removes in-stent thrombus and restores flow. It could also potentially reduce the need for additional reintervention. Another medical personnel commented that with the availability of the Triever16 Curve catheter, a wider VTE patient population will likely be served.
Industry Prospects
Per a report by Future Market Insights, the global VTE treatment market was estimated to be $1.53 billion and is anticipated to reach $1.91 billion in 2029 at a CAGR of 3.7%. Factors like the increasing pervasiveness of cardiovascular diseases and cancer due to the adoption of smoking and increasing obesity rate, the rising elderly population and technological advancements are likely to drive the market.
Given the market potential, the commercial launches are likely to provide a significant boost to Inari Medical’s business globally.
Notable Developments
Last month, Inari Medical announced planned enrollment of the PEERLESS II trial, its third randomized controlled trial (RCT) in VTE. PEERLESS II is a prospective, global, multi-center RCT comparing the outcomes of intermediate-risk pulmonary embolism (PE) patients treated with the FlowTriever system versus anticoagulation alone.
The same month, Inari Medical announced its first-quarter 2023 results, wherein it registered a solid uptick in its year-over-year revenues. Management also confirmed the receipt of FDA clearances for several new products that are expected to drive market expansion while safeguarding the company from both existing and future competition.
In March, Inari Medical announced positive results from the FLAME study in high-risk/massive PE. FLAME is the largest prospective study of interventional treatment in high-risk PE, a patient population with a historical mortality rate of 25-50%.
Price Performance
Shares of the company have lost 6.7% in the past year against the industry’s 4.3% rise and the S&P 500's 6.4% growth.
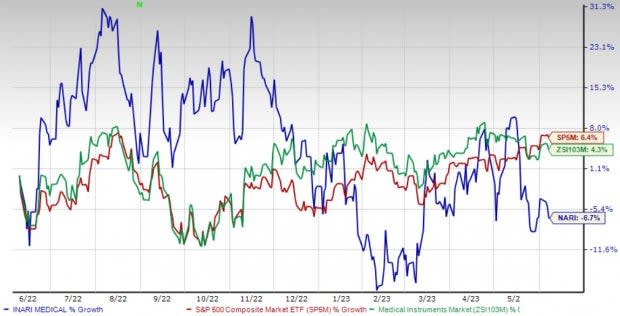
Image Source: Zacks Investment Research
Zacks Rank & Key Picks
Currently, Inari Medical carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the broader medical space are Hologic, Inc. HOLX, Merit Medical Systems, Inc. MMSI and Boston Scientific Corporation BSX.
Hologic, carrying a Zacks Rank #2 (Buy) at present, has an estimated growth rate of 5.1% for fiscal 2024. HOLX’s earnings surpassed estimates in all the trailing four quarters, the average being 27.3%. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Hologic has gained 6.9% compared with the industry’s 4.3% rise in the past year.
Merit Medical, carrying a Zacks Rank #2 at present, has an estimated long-term growth rate of 11%. MMSI’s earnings surpassed estimates in all the trailing four quarters, the average surprise being 20.2%.
Merit Medical has gained 45.9% compared with the industry’s 10.5% rise over the past year.
Boston Scientific, carrying a Zacks Rank #2 at present, has an estimated long-term growth rate of 11.5%. BSX’s earnings surpassed estimates in two of the trailing four quarters and missed in the other two, the average surprise being 1.9%.
Boston Scientific has gained 33.5% against the industry’s 29.8% decline over the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Boston Scientific Corporation (BSX) : Free Stock Analysis Report
Hologic, Inc. (HOLX) : Free Stock Analysis Report
Merit Medical Systems, Inc. (MMSI) : Free Stock Analysis Report
Inari Medical, Inc. (NARI) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 