Insights on the Clinical Trials Site Management Organizations Global Market to 2030 - Increasing Rate of Clinical Trials Outsourcing by Companies is Driving Growth

Global Clinical Trials Site Management Organizations Market
Dublin, May 30, 2022 (GLOBE NEWSWIRE) -- The "Clinical Trials Site Management Organizations Market Size, Share & Trends Analysis Report by Clinical Trail Services/Components, by Phase, by Therapeutic Areas (Oncology, Cardiology, CNS), by Region, and Segment Forecasts, 2022-2030" report has been added to ResearchAndMarkets.com's offering.
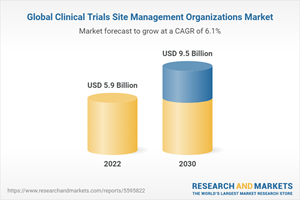
The global clinical trials site management organizations market size is expected to reach USD 9.5 billion by 2030. The market is expected to expand at a CAGR of 6.1% from 2022 to 2030.
Site management organizations (SMOs) are essential elements of pharmaceutical and biopharmaceutical companies. Such organizations help to limit the burden associated with clinical research. SMOs offer several services including patient enrollment services, addressing clinical trial location-specific study activities, hiring study staff, and monitoring clinical studies site operations. Improved technological use in integrated site networks and clinical trial services, increasing trend of outsourced clinical services, and growing clinical trial activities globally owing to the high burden of chronic and infectious diseases, are few of the factors driving the market.
Technology has improved efficiencies at the site level by improving metrics such as on-site identification, selection, and performance, as well as throughout the patient spectrum by analyzing recruitment, enrollment, selection, retention, and compliance measures at sites.
Furthermore, technical improvements have resulted in enhanced biostatistics and data analytics analysis to better evaluate a drug's feasibility early in the development phase. As a result of the COVID-19 pandemic, several organizations were forced to halt operations while others were forced to completely shut down. However, the SMOs execute multi-eccentric trials effectively to save the firm money and time.
Manual processes and paperwork are replaced by digital technologies to limit the negative impact of the pandemic on the business. Due to this, Site Management Organization facilitates detailed documentation while also streamlining the procedure in any clinical research, even when physical contact is nearly impossible to prevent viral spread. SMO's efficient follow-up skills along with the adoption of virtual technologies to conduct clinical research have significantly decreased the time required to recruit patients.
As new virus strains are being discovered in different areas of the world, research would remain a top focus. SMOs would be required by health and biotech companies for data collection, participant or patient safety, patient recruitment, more accurate doctor contact information, and other tasks. Hence, such factors are supporting the rebound of revenues across the market during 2021.
Clinical Trials Site Management Organizations Market Report Highlights
The project management segment dominated the market with a revenue share of 27.6% in 2021, due to the fact that it is required to ensure that clinical trials are set up, enrolled, reported on time, and conducted within the budget.
The phase III segment accounted for the largest revenue share of 54.0% in 2021. This growth is attributed to the fact that phase III trials are often the largest and involve thousands of participants and are the most expensive ones.
Based on therapeutic area, the oncology segment accounted for the largest share of 25.2% in 2021, due to the high global prevalence of cancer, which is generating demand for drugs and thus increasing its market share.
Asia Pacific led the market in 2021 and is projected to witness the fastest CAGR of 6.8% during the forecast years, as cost-effective strategic solutions provided by SMOs could reduce timelines, as in changing market for clinical trials, fast recruitment, and a huge pool of patients are some of the prerequisites.
Key Topics Covered:
Chapter 1 Methodology and Scope
Chapter 2 Executive Summary
Chapter 3 Clinical Trial Site Management Organizations (SMO) Market: Variables, Trends, & Scope
3.1 Market Segmentation and Scope
3.2 Market Dynamics
3.2.1 Market Driver Analysis
3.2.1.1 Growth in the number of clinical Studies
3.2.1.2 Rising investment in R&D by the pharmaceutical companies.
3.2.1.3 Increasing rate of clinical trials outsourcing by pharmaceutical and biopharmaceutical companies.
3.2.2 Market Restraint Analysis
3.2.2.1 High cost of clinical studies
3.2.2.2 Presence of significant CROs offering site management services possesses the threat to steal its market share
3.3 Penetration & Growth Prospect Mapping
3.4 COVID-19 Impact on the Market
3.5 Major Deals and Strategic Alliances Analysis
3.6 Clinical Trial Site Management Organizations (SMO)s: Market Analysis Tools
3.6.1 Industry Analysis-Porter's
3.6.2 PESTEL Analysis
Chapter 4 Clinical Trial Site Management Organizations (SMO) Market: Clinical Trial Services/ Components Segment Analysis
4.1 Clinical Trial Site Management Organizations (SMO) Market: Definition & Scope
4.2 Clinical Trial Site Management Organizations (SMO): Market Share Analysis, 2021 & 2030
4.3 Site management
4.3.1 Site management Market, 2018-2030 (USD Million)
4.4 Project management
4.4.1 Project management market, 2018-2030 (USD Million)
4.5 Onsite monitoring
4.5.1 Onsite monitoring market, 2018-2030 (USD Million)
4.6 Regulatory
4.6.1 Regulatory Market, 2018-2030 (USD Million)
4.7. Others
4.7.1 Others market, 2018-2030 (USD Million)
Chapter 5 Clinical Trial Site Management Organizations (SMO) Market: Phase Analysis
5.1 Clinical Trial Site Management Organizations (SMO) Market: Definition & Scope
5.2 Clinical Trial Site Management Organizations (SMO) : Market Share Analysis, 2021 & 2030
5.3 Phase I
5.3.1 Phase I Market, 2018-2030 (USD Million)
5.4. Phase II
5.4.1 Phase II market, 2018-2030 (USD Million)
5.5 Phase III
5.5.1. Phase III market, 2018-2030 (USD Million)
5.6 Phase IV
5.6.1. Phase IV market, 2018-2030 (USD Million)
Chapter 6 Clinical Trial Site Management Organizations (SMO) Market: Therapeutic Area Analysis
6.1 Clinical Trial Site Management Organizations (SMO) Market: Definition & Scope
6.2 Clinical Trial Site Management Organizations (SMO): Market Share Analysis, 2021 & 2030
6.3 Oncology
6.3.1 Oncology Market, 2018-2030 (USD Million)
6.4. Cardiology
6.4.1 Cardiology market, 2018-2030 (USD Million)
6.5 CNS
6.5.1. CNS market, 2018-2030 (USD Million)
6.6 Pain management
6.6.1. Pain management market, 2018-2030 (USD Million)
6.7 Endocrine
6.7.1. Endocrine market, 2018-2030 (USD Million)
6.8 Others
6.8.1. Others market, 2018-2030 (USD Million)
Chapter 7 Clinical Trial Site Management Organizations (SMO) Market: Regional Analysis
Chapter 8 Company Profiles
8.1 Company Profiles
8.1.1 Clinedge
8.1.1.1 Company overview
8.1.1.2 Financial performance
8.1.1.3 Service benchmarking
8.1.1.4 Strategic initiative
8.1.2 WCG
8.1.2.1 Company overview
8.1.2.2 Financial performance
8.1.2.3 Service benchmarking
8.1.3 ClinChoice
8.1.3.1 Company overview
8.1.3.2 Financial performance
8.1.3.3 Service benchmarking
8.1.3.4 Strategic initiatives
8.1.4 Access Clinical Research
8.1.4.1 Company overview
8.1.4.2 Financial performance
8.1.4.3 Product benchmarking
8.1.4.4 Strategic initiatives
8.1.5 FOMAT Medical Research INC.
8.1.5.1 Company overview
8.1.5.2 Product benchmarking
8.1.5.3 Strategic initiatives
8.1.6 SGS
8.1.6.1 Company overview
8.1.6.2 Product benchmarking
8.1.6.3 Strategic initiatives
8.1.7 KV Clinical
8.1.7.1 Company overview
8.1.7.2 Financial performance
8.1.7.3 Product benchmarking
8.1.7.4 Strategic initiatives
8.1.8 SMO-Pharmina
8.1.8.1 Company overview
8.1.8.2 Financial performance
8.1.8.3 Product benchmarking
8.1.9 Xylem Clinical Research
8.1.9.1 Company overview
8.1.9.2 Financial performance
8.1.9.3 Product benchmarking
8.1.10 Aurum Clinical Research
8.1.10.1 Company overview
8.1.10.2 Financial performance
8.1.10.3 Product benchmarking
8.1.10.4 Strategic Initiatives
For more information about this report visit https://www.researchandmarkets.com/r/6bgisu
Attachment
CONTACT: CONTACT: ResearchAndMarkets.com Laura Wood, Senior Press Manager press@researchandmarkets.com For E.S.T Office Hours Call 1-917-300-0470 For U.S./CAN Toll Free Call 1-800-526-8630 For GMT Office Hours Call +353-1-416-8900


 Yahoo Finance
Yahoo Finance 
