Ionis' (IONS) Partner to Begin New Huntington's Disease Study
Ionis Pharmaceuticals, Inc. IONS announced that its partner, Roche Holding AG RHHBY, is designing a new phase II study to evaluate its pipeline candidate, tominersen, in Huntington's disease (“HD”), a rare neurodegenerative disorder. Currently, there is no approved therapy that can treat the underlying cause of HD.
Swiss pharma giant Roche licensed tominersen from Ionis in December 2017.
In March last year, Roche stopped dosing in a phase III study of tominersen in HD. The decision, which was taken on the recommendation of an Independent Data Monitoring Committee (iDMC), was based on a pre-planned review of the data from the phase III GENERATION HD1 study by said iDMC. Ionis’ stock price plunged heavily on the news back then.
No new safety signals were identified in the review for the candidate. However, the iDMC’s recommendation was based on the candidate’s potential benefit/risk profile for study participants. Roche also paused an open-label extension study (GEN-EXTEND) of tominersen back then.
Roche is currently in early stages of planning the phase II study, which is designed to evaluate different doses of tominersen in a younger adult patient population. The company will provide more updates on this new study at an upcoming medical conference.
Per the latest press release, an exploratory post-hoc analysis of the phase III GENERATION HD1 study showed that tominersen might benefit younger adult patients who have a much lower disease burden. However, these results need to be confirmed through a placebo-controlled study. The GENERATION HD1 study is currently ongoing without dosing and is expected to be completed in March/April 2022.
Shares of Ionis have plunged 46% in the past year compared with the industry’s decrease of 27.6%.
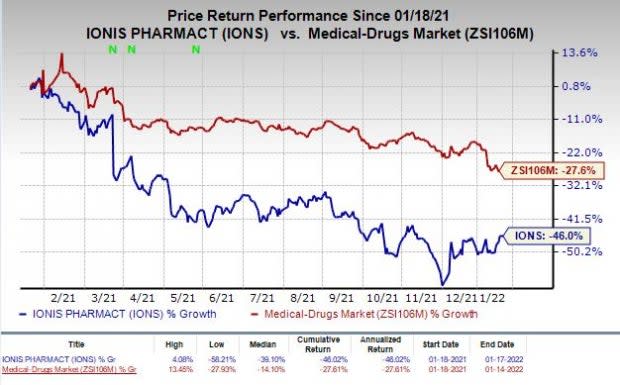
Image Source: Zacks Investment Research
Like the GENERATION HD1 study, the open-label extension study (GEN-EXTEND) of tominersen for participants coming from any Roche HD study is also ongoing without dosing, and is also expected to be completed in March/April 2022. A phase I study (GEN-PEAK) is being carried out to better understand the pharmacokinetics of tominersen.
Other the Roche, Ionis has collaboration deals with leading drugmakers/biotech companies like Biogen BIIB and Novartis NVS, to name a couple, for developing and marketing its medicines. The partnership deals provide it with funds in the form of license fees, upfront payments and milestone payments to invest in its pipeline development.
Biogen has in-licensed Spinraza from Ionis, which is approved for treating SMA. Biogen is responsible for commercializing the drug globally. Ionis receives royalties from Biogen on Spinraza’s sales.
Biogen has expanded its collaboration with Ionis to identify new gene therapies for the treatment of SMA as well as a broad range of neurological diseases. Some candidates that Ionis is developing in partnership with Biogen are ION541 for amyotrophic lateral sclerosis or ALS (phase II), tofersen for SOD1-ALS (phase III) and ION859 for Parkinson’s disease (phase II).
Ionis is developing pelacarsen in partnership with Novartis in late-stage studies for cardiovascular disease due to elevated Lp(a) levels. In 2019, Novartis in-licensed pelacarsen from Ionis.
Novartis is now responsible for conducting and funding the development of pelacarsen, including a global phase III Lp(a) HORIZON cardiovascular outcome study.
Zacks Rank
Ionis currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Ionis Pharmaceuticals, Inc. (IONS) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 