LabCorp (LH) Gets FDA Nod for VirSeq SARS-CoV-2 NGS Test
Laboratory Corporation of America Holdings or LabCorp LH recently gained FDA emergency use authorization (EUA) for its VirSeq SARS-CoV-2 NGS (next-generation sequencing) Test on the PacBio Sequel II sequencing system. The latest regulatory authorization demonstrates LabCorp’s ability to respond promptly during the pandemic while highlighting the strength of its scientists, the value of its technology and its continued focus on innovation.
The FDA EUA for the SARS-CoV-2 NGS Test will likely fortify LabCorp’s COVID-19 Testing (consisting of COVID-19 PCR and antibody testing) business.
Few Words on VirSeq SARS-CoV-2
The VirSeq SARS-CoV-2 NGS Test can detect certain SARS-CoV-2 strains in patient samples. It is the first test approved for identifying and distinguishing between SARS-CoV-2 Phylogenetic Assignment of Named Global Outbreak (PANGO) lineages, which are genetic variations in circulating virus strains. Health care providers can employ the test if they believe that strain-specific information can guide appropriate treatment decisions based on a patient’s medical history and other diagnostic data.
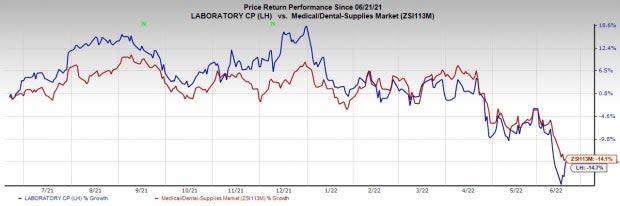
Image Source: Zacks Investment Research
The test is not intended to be utilized in making a primary SARS-CoV-2 infection diagnosis, confirming the presence of infection or identifying specific SARS-CoV-2 genomic mutations.
Industry Prospects
Per a report by Grand View Research, the global COVID-19 diagnostics market is expected to see a CAGR of 7.7% during 2022-2030. Market expansion can be attributed to increased government initiatives for mass testing, a growing patient population, rising need for speedy diagnostics and the absence of specific drugs.
Given the market prospects, the regulatory clearance for LabCorp’s VirSeq SARS-CoV-2 NGS test is opportune.
Notable Developments
In June 2022, LabCorp introduced a new testing program, sponsored by Eli Lilly and Company LLY, to help advanced non-small cell lung cancer (NSCLC) patients and their physicians make detailed treatment and care management decisions based on comprehensive genomic insights. The program will leverage LabCorp’s OmniSeq INSIGHT test to offer comprehensive genomic and immune profiling for cases that match the eligibility criteria. Management at Eli Lilly and Company’s feels that the latest agreement to bring OmniSeq INSIGHT tests to more patients will demonstrate the importance of precision medicine.
In May 2022, the company announced the launch of a test that assesses Lymphocyte-activation gene 3 (LAG-3) expression levels by immunohistochemistry in tumor tissue, thereby expanding treatment options for skin cancer. The test is available for use in both clinical trials and the care and treatment of patients. It was developed by LabCorp Drug Development for a clinical trial that is studying dual checkpoint inhibitors, including LAG-3 immunotherapy.
The company also announced the launch of an at-home collection kit for diabetes screening, which measures hemoglobin A1c (HbA1c) from a small blood sample. With the introduction of the kit, consumers can access the diabetes risk test kit and other health and wellness tests through “Labcorp OnDemand.” The Labcorp OnDemand Diabetes Risk test utilizes a dried blood technology to provide a snapshot of the body’s average blood sugar levels over time.
Share Price Performance
The stock has underperformed its industry in the past year. It has declined 14.7% compared with the industry’s 14.1% fall.
Zacks Rank and Key Picks
Currently, LabCorp carries a Zacks Rank #3 (Hold).
Two better-ranked stocks in the broader medical space are Alkermes plc ALKS and AMN Healthcare Services, Inc. AMN.
Alkermes has an estimated long-term growth rate of 25.1%. Alkermes’ earnings surpassed estimates in the trailing four quarters, the average surprise being 350.5%. It currently sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Alkermes has outperformed the industry in the past year. ALKS has gained 13.3% against the industry’s 44.1% decline in the said period.
AMN Healthcare has a long-term earnings growth rate of 1.1%. The company surpassed earnings estimates in the trailing four quarters, delivering an average surprise of 15.6%. It currently flaunts a Zacks Rank #1.
AMN Healthcare has outperformed its industry in the past year. AMN has gained 4.2% against the industry’s 54.5% fall.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 