Ligand (LGND) Acquires Certain Assets of Novan for $12.2M
Ligand Pharmaceuticals LGND announced that it has completed the acquisition of certain assets of Novan, Inc. for $12.2 million.
As a result, Ligand now has full ownership rights to berdazimer gel, all assets related to the NITRICIL drug delivery technology platform and rights to the Bayer-partnered Sitavig program. The remaining assets of Novan have been sold to other parties.
The completion of this transaction follows Ligand’s initial offer in July 2023 to acquire all of Novan’s assets for $15 million. The offer was made after Novan filed for Chapter 11 reorganization of the U.S. Bankruptcy Code. Alongside the offer, Ligand had also provided $15 million in debtor-in-possession (“DIP”) financing to Novan.
Following the completion of the transaction, the $12.2 million bid was adjusted against the DIP financing provided by Ligand and the balance payment, along with interest, has been received by the company.
Coherent with its business strategy, LGND will most likely seek to out-license or sell these acquired assets. Management expects this deal to maximize its shareholder value.
Ligand had previously acquired milestone and royalty rights to berdazimer gel in 2019. A new drug application (“NDA”) filing is under FDA review seeking approval for berdazimer gel as a potential treatment for molluscum contagiosum infection. A final decision from the regulatory body is expected by Jan 5, 2024.
Year to date, shares of Ligand have lost 8.0% compared with the industry’s 15.9% decline.
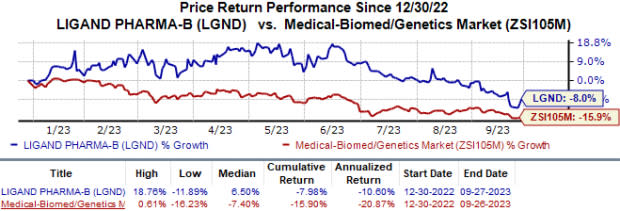
Image Source: Zacks Investment Research
Berdazimer gel is the first self-application topical treatment for molluscum contagiosum infection, a highly contagious viral skin disease. Ligand believes in the potential of berdazimer gel to get approved by the FDA. Currently, there are no FDA-approved prescription drug treatment options for molluscum contagiosum infection, representing a severe unmet medical need.
Recently, Ligand has been seeking business opportunities aimed to help accelerate its profitability while lowering its infrastructure costs at the same time.
Earlier this month, Ligand signed an agreement to merge its subsidiary Pelican Technology Holdings, Inc., with Primordial Genetics to create a new stand-alone private company focused on synthetic biology called ‘Primrose Bio’. After this merger, Ligand will own 49.9% of the newly merged entity and retain all the existing commercial royalties related to Pelican (or Pfenex) Expression technology.
A positive outcome of the divestiture, Ligand also raised its adjusted diluted EPS to $5.10-$5.25 from the previously issued guidance of $4.85-$5.00.
Ligand acquired the Pelican technology following the acquisition of Pfenex in 2020. Since the closure of the acquisition, the company has five approved medicines that utilize the Pelican technology, including rights to Jazz Pharmaceuticals’ JAZZ Rylaze and Merck’s MRK Vaxneuvance and V116 vaccines, among others.
The FDA approves Jazz’s Rylaze to treat acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL) indications in patients aged one month and older. Due to strong demand, sales from the JAZZ-marketed drug have been rising consistently in recent quarters. Last week, the European Commission approved Rylaze for similar indications in the European Union (“EU”). Jazz will market the drug in the EU under the brand name Enrylaze.
Merck’s Vaxneuvance is a 15-valent pneumococcal conjugate vaccine approved by the FDA for use in individuals six weeks and older. The Merck vaccine is also approved for similar use in the European Union.
In July, Merck reported positive top-line results from two late-stage studies evaluating V116, an investigational 21-valent pneumococcal conjugate vaccine, in vaccine-naïve and previously vaccinated individuals. Both studies conducted by Merck achieved their key immunogenicity and safety endpoints.
Ligand Pharmaceuticals Incorporated Price
Ligand Pharmaceuticals Incorporated price | Ligand Pharmaceuticals Incorporated Quote
Zacks Rank
Ligand currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Jazz Pharmaceuticals PLC (JAZZ) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 