Moderna’s COVID-19 vaccine nearly 95% effective in trials
WATCH: Moderna says it’s vaccine appears to be 94.5% effective
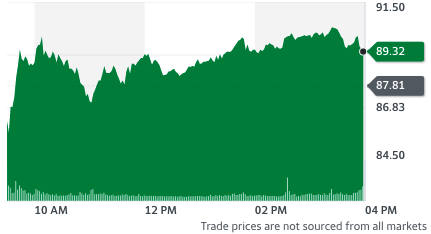
US biotech company Moderna (MRNA), announced on Monday that its COVID-19 vaccine candidate has shown efficacy of 94.5% during the phase 3 trials of 30,000 participants in the US.
Moderna’s share price surged above 8% after US markets opened.
The company’s announcement comes exactly a week after Germany’s BioNTech (BNTX) and its US partner Pfizer (PFE) said their vaccine was around 90% effective in phase 3 trials, based on preliminary data.
Both companies are working on messenger RNA (mRNA) vaccines. Unlike traditional vaccines, which work by putting weak or inactivated doses of a virus or bacteria into the body to make the immune systems produce antibodies, mRNA vaccines transmit a genetic code to cells telling them to produce a protein, which in turn activates the immune system.
READ MORE: BioNTech founder on COVID-19 vaccine trial results: 'An important step for the world'
Moderna’s vaccine can be stored at between 2C and 8C, which is the temperature of normal fridges, making it easier to store than the Pfizer/BioNTech vaccine, which needs to be kept mostly at -70C.
“This is a pivotal moment in the development of our COVID-19 vaccine candidate,” said Moderna CEO Stéphane Bancel in a press statement.
“Since early January, we have chased this virus with the intent to protect as many people around the world as possible. This positive interim analysis from our Phase 3 study has given us the first clinical validation that our vaccine can prevent COVID-19 disease.”
READ MORE: Coronavirus: J&J’s Janssen to begin UK vaccine trials.
Moderna said that it had submitted its results to the European regulator, the EMA, to begin its rolling review. It also said it will now apply to the US Food and Drug Administration for emergency-use authorisation.
Moderna expects to deliver 20 million doses in the US by the end of this year, and is targeting production up to 500 million doses in 2021, to be available globally. Combined with what Pfizer is planning to deliver, the US could have 60 million doses available to start vaccinating the population by the end of 2021.
However, UK does not have any prior order placed for Moderna’s COVID-19 vaccines as yet. The government said last week that it had ordered 10 million doses of the BioNTech vaccine, and in August reached a deal with US-based Novavax (NVAX) to purchase 60 million doses and with Johnson & Johnson (JNJ) for 30 million doses.
The European Commission said today that it was still negotiating a vaccine supply agreement with Moderna. It signed an agreement for 300 million doses of BioNTech/Pfizer’s vaccine last week.

 Yahoo Finance
Yahoo Finance 