Mylan (MYL) to Get CRL Again for Generic Advair from FDA
Shares of Mylan N.V. MYL are down after the company announced that the FDA will not approve its Abbreviated New Drug Application for the generic version of GlaxoSmithKline’s GSK asthma drug, Advair Diskus, yet again.
The agency found minor deficiencies in the application, which will be relayed to the company in a Complete Response Letter (CRL) to be issued on June 27, 2018.
The news comes as a disappointment for Mylan. Last year, the FDA had issued a CRL for it, as well. A probable approval would have boosted Mylan’s generic portfolio immensely, given the potential market for the same.
The CRL also puts a question mark on Mylan’s 2018 guidance and the company is expected to update on the same.
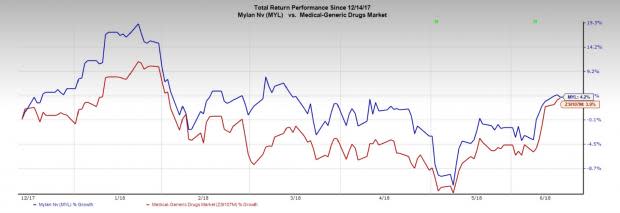
Mylan’s stock has gained 4.2% in the last six months compared with the industry’s gain of 3.9%.
After a challenging 2017, things were looking up for Mylan in 2018 with recent approvals, which are expected to help the company combat the persistent decline in EpiPen sales.
Mylan’s biosimilar portfolio got a boost with the recent FDA approval of Fulphila, a biosimilar of Neulasta. The company had earlier won the FDA approval of Ogivri, a biosimilar of Herceptin.
Earlier, Mylan received a major boost with the FDA approval for a generic version of Teva’s TEVA Copaxone 40mg. Being one of the first filers, it will also enjoy 180 days of exclusivity. The company has more than 30 submissions planned in 2018.
Hence, the approval of generic Advair would have further propelled the company’s broad portfolio of biosimilars and generics.
Meanwhile, Mylan is not the only company whose ANDA has been rejected by the FDA. Earlier in 2018, Swiss pharma giant Novartis’ NVS generic arm Sandoz also got a CRL from the FDA for its generic version of Advair. Sandoz is in the process of addressing the issues identified in the CRL and it does not expect to launch the generic before 2019 end. Hikma Pharmaceuticals also received a CRL from the FDA in 2017 for its generic version of Advair.
On the other hand, the delay in approval of generic version of Advair bodes well for Glaxo. Concurrent with the first-quarter results, Glaxo stated that it expects Advair sales of around £750 million in 2018, assuming a mid-year introduction of a substitutable generic competitor to Advair in the United States.
Zacks Rank
Mylan carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank stocks here.
5 Medical Stocks to Buy Now
Zacks names 5 companies poised to ride a medical breakthrough that is targeting cures for leukemia, AIDS, muscular dystrophy, hemophilia and other conditions.
New products in this field are already generating substantial revenue and even more wondrous treatments are in the pipeline. Early investors could realize exceptional profits.
Click here to see the 5 stocks >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Teva Pharmaceutical Industries Ltd. (TEVA) : Free Stock Analysis Report
Mylan N.V. (MYL) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 
