Novavax (NVAX) Seeks FDA Authorization for COVID-19 Booster Dose
Novavax NVAX filed a regulatory application with the FDA, which seeks to expand the emergency use authorization (EUA) granted its protein-based COVID-19 vaccine called Novavax COVID-19 Vaccine, Adjuvanted (NVX-CoV2373).
The EUA seeks label expansion for the use of Novavax’s vaccine as a homologous and heterologous booster dose in adults (aged 18 years and older).
Though the vaccine was previously granted EUA in the United States as a primary two-dose regimen for use in adults last month, a vast majority of this population has already been immunized with an approved primary regimen of a vaccine developed by Pfizer PFE/BioNTech BNTX, Moderna MRNA and J&J.
Based on updated data (as of Aug 10, 2022) from the Centers for Disease Control and Prevention (CDC), 90% of the country’s adult population received at least one dose of a vaccine, while 77.2% of the U.S. adult population has been fully vaccinated.
If authorized for a booster dose, Novavax can target a larger/wider segment of the adult population in the U.S. market who have already been vaccinated but are yet to receive a booster dose of the vaccine. Unlike the primary regimen of vaccines, which require receiving doses of the same vaccine, a booster dose of a different vaccine can be administered. The CDC data (as of Aug 10, 2022) indicates that only 51.4% of the country’s adult population has received a third/booster dose of an authorized vaccine.
In the year-to-date period, shares of Novavax have plunged 70.4% compared with the industry’s 17.9% decline.
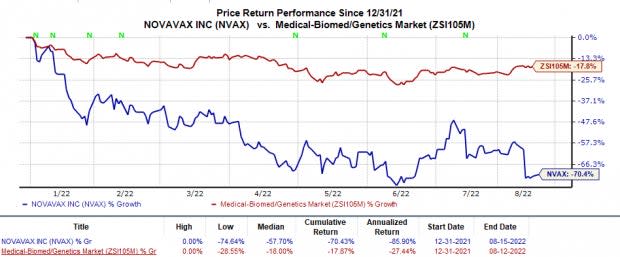
Image Source: Zacks Investment Research
Novavax’s FDA filing seeking approval for the booster dose is supported by data from the phase III PREVENT-19 study (conducted in the United States and Mexico) and phase II COV-BOOST (conducted in the United Kingdome), which evaluated a booster dose of NVX-CoV2373 in healthy adult participants. Data from these studies showed that a third dose of the vaccine generated a higher antibody response in the study participants compared with the data observed in late-stage studies evaluating the primary two-dose regimen of NVX-CoV2373. The vaccine also generated a higher immune response when used as a heterologous booster dose.
Novavax’s COVID-19 vaccine is already authorized for use in more than 40 countries, including Australia, Canada, European countries and India. NVAX is currently marketing two versions of NVX-CoV2373 — one in partnership with the Serum Institute of India under the trade name Covovax, and the other produced by itself and marketed under the trade name Nuvaxovid.
The COVID-19 vaccine market in the United States is dominated by Modernaand Pfizer/BioNTech, which market mRNA-based vaccines.
While Novavax is yet to tap the potential of the U.S. market, Moderna and Pfizer/BioNTech are currently way ahead of the curve. The vaccines they developed dominate the U.S. market and are the only jabs that have received full approval for use in the country. While Moderna’s vaccine is approved for use in adults 18 years and older, the Pfizer/BioNTech vaccine is approved for use in individuals 12 years and older. The vaccines are also authorized for emergency use in individuals aged six months and above in the United States.A third and fourth booster dose of Moderna and Pfizer/BioNTech vaccines have also been authorized for use in certain age groups by the FDA.
In a separate press release, Novavax also announced that it had submitted a regulatory application in Taiwan seeking to expand the EUA for its COVID-19 vaccine for use in adolescents aged between 12 and 17 years. The vaccine is currently authorized for use in adults since June 2022.
Novavax, Inc. Price
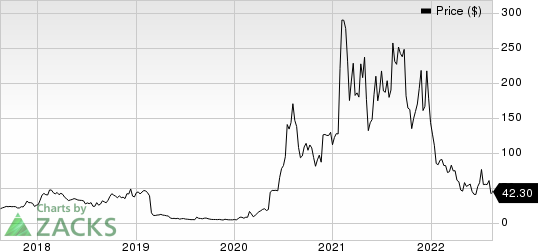
Novavax, Inc. price | Novavax, Inc. Quote
Zacks Rank
Novavax currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 