Pfizer's Pneumococcal Vaccine Shows Potential in Infants
Pfizer Inc. PFE announced encouraging data from a phase II study evaluating its 20-valent pneumococcal conjugate vaccine (20vPnC) candidate, PF-06482077, in healthy infants.
The proof-of-concept phase II study is a four-dose series evaluating the safety and immunogenicity of PF-06482077 for the prevention of invasive disease and otitis media in healthy infants caused by Streptococcus pneumoniae serotypes contained in the vaccine. Preliminary data from the initial three doses of PF-06482077 in infants demonstrated a similar safety profile to Pfizer’s blockbuster 13-valent pneumococcal conjugate vaccine, Prevnar 13.
Please note that the company’s 20vPnC candidate includes all the 13 serotypes contained in Prevnar 13 along with seven additional serotypes. Data from the phase II study showed that the candidate led to immune responses for all 20 serotypes.
The company stated that the findings are encouraging enough to support PF-06482077’s development in infants to late stage. It will start discussions with regulatory authorities for initiating a phase III study in infants following the availability of data on the fourth dose.
Pfizer is also developing PF-06482077 in adult patients. Concurrently with this press release, it announced that enrollment in three phase III studies evaluating the candidate in adult patients has been completed. The studies are evaluating the candidate in adult patients as a preventive treatment for invasive disease and pneumonia caused by Streptococcus pneumoniae serotypes The company plans to submit a biologics license application seeking approval of the candidate in adults by the end of 2020, following the successful completion of late-stage studies.
PF-06482077 was granted Breakthrough Therapy designation as a vaccine for adults in 2018. It also enjoys Fast Track designation.
Pfizer’s shares have declined 15.7% so far this year compared with the industry decrease of 0.6%.

The company states that the additional seven serotypes contained in PF-06482077 compared to Prevnar 13 are associated with high case-fatality rates, antibiotic resistance and/or meningitis. Prevnar 13 generated sales of $1.18 billion in the first six months of 2019. With additional seven serotypes contained in PF-06482077, it has potential to cater to an expanded patient population and, if approved, should bring significant sales for Pfizer.
A few other companies are also developing pneumococcal conjugate vaccines including Merck’s MRK 15-valent pneumococcal conjugate vaccine candidate, V114.
Pfizer Inc. Price
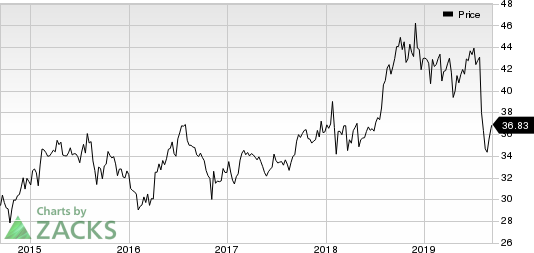
Pfizer Inc. price | Pfizer Inc. Quote
Zacks Rank & Stocks to Consider
Pfizer currently carries a Zacks Rank #4 (Sell).
A couple of better-ranked large-cap pharma stocks include Roche Holding AG RHHBY and Eli Lilly LLY. While Roche sports a Zacks Rank #1 (Strong Buy), Lilly carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Shares of Roche have gained 10.4% this year so far. Earnings estimates for 2019 have risen 1.2% while that for 2020 have increased 2% over the past 60 days.
Lilly’s earnings estimates have increased 1.1% and 0.6%, respectively, for 2019 and 2020 over the past 60 days.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 