Pfizer's sBLA for Bavencio Gets FDA Nod, Daurismo Gets EU Nod
Pfizer Inc. PFE along with Germany-based partner Merck KGaA announced that the FDA has approved a supplemental biologics license application (sBLA) for their PD-L1 inhibitor Bavencio (avelumab). The drug is now approved as a first-line maintenance treatment of patients with locally advanced/metastatic urothelial carcinoma (UC), a form of bladder cancer. Notably, the disease in this cohort did not progress with first-line platinum-containing chemotherapy.
The FDA nod was based on data from the phase III JAVELIN Bladder 100 study, which evaluated Bavencio plus best supportive care (BSC) in the given patient population.
Results from the study demonstrated a significant 7.1 month improvement in median overall survival (OS) with Bavencio as first-line maintenance treatment plus BSC compared to BSC alone. This statistically significant improvement in OS represents a 31% reduction in the risk of death in the overall population.
Bavencio is already approved for patients with locally advanced or metastatic UC who have disease progression during or following platinum-containing chemotherapy or in other words for second-line use.
Additionally, Bavnecio is approved for use in combination with Pfizer’s Inlyta for first-line treatment of advanced kidney cancer and for Merkel cell carcinoma. Although Bavencio is currently approved for three relatively smaller indications, it might emerge as a key long-term driver for Pfizer on future label-expansion approvals.
However, Bavencio faces stiff competition from other approved PD-L1 inhibitors in the market, such as Merck’s MRK blockbuster drug Keytruda (pembrolizumab), Bristol-Myers’ BMY Opdivo (nivolumab) and AstraZeneca’s AZN Imfinzi (durvalumab).
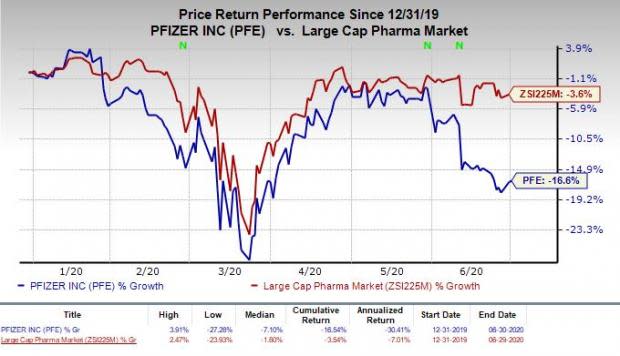
Shares of Pfizer have declined 16.6% in the year so far compared with the industry’s decrease of 3.6%.
In a separate press release, Pfizer announced that the European Commission has approved its once-daily oral medicine Daurismo (glasdegib) in combination with low-dose cytarabine (LDAC) for the treatment of newly-diagnosed acute myeloid leukemia (AML) in adult patients who are not candidates for standard chemotherapy.
The nod in the EU was based on outcomes from the phase II BRIGHT 1003 study. More than 50% of patients treated with the Daurismo combo experienced a reduction in the risk of death compared to those having received LDAC alone.
In November 2018, Daurismo in combination with LDAC was approved in the United States for newly diagnosed AML patients falling under the age group of 75 years and above or who cannot take intensive chemotherapy.
Zacks Rank
Pfizer currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Biggest Tech Breakthrough in a Generation
Be among the early investors in the new type of device that experts say could impact society as much as the discovery of electricity. Current technology will soon be outdated and replaced by these new devices. In the process, it’s expected to create 22 million jobs and generate $12.3 trillion in activity.
A select few stocks could skyrocket the most as rollout accelerates for this new tech. Early investors could see gains similar to buying Microsoft in the 1990s. Zacks’ just-released special report reveals 8 stocks to watch. The report is only available for a limited time.
See 8 breakthrough stocks now>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Merck Co., Inc. (MRK) : Free Stock Analysis Report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
To read this article on Zacks.com click here.

 Yahoo Finance
Yahoo Finance 