Pharma Stock Roundup: PFE Booster Jab Gets FDA Nod, JNJ's New Data on COVID-19 Shot
This week was all about booster doses of COVID-19 vaccines. The FDA granted emergency use approval (EUA) to Pfizer PFE/BioNTech’s BNTX COVID-19 vaccine booster shots for older adults and high-risk people. J&J’s JNJ new data showed that a booster dose of its vaccine generated 94% efficacy against mild-to-severe COVID-19 in the United States.
Recap of the Week’s Most Important Stories
FDA Approves Pfizer’s COVID-19 Booster for Older Adults and High-Risk Groups: The FDA granted EUA to a booster dose of Pfizer/BioNTech’s mRNA-based COVID-19 vaccine, Comirnaty, for individuals 65 years and older and also those in high-risk groups. However, the FDA did not approve the booster dose for the entire population (people 16 years of age and older) for which Pfizer/BioNTech were seeking approval.
The FDA’s decision was more or less in line with the recommendation issued by its Vaccines and Related Biological Products Advisory Committee (“VRBPAC”) last week. The VRBPAC voted unanimously, recommending that the FDA grant EUA to Comirnaty booster dose for individuals 65 years and older and those at high risk of severe COVID-19. However, the panel voted against approving the booster for the general population.
Pfizer’s pivotal phase II/III study showed that its COVID-19 vaccine was safe, well-tolerated, and initiated robust neutralizing antibody responses in children five to 11 years of age. The companies plan to submit the data to the FDA and other regulators soon.
Pfizer/BioNTech announced plans to provide the U.S. government with 500 million additional doses of Comirnaty at not-for-profit price for donation to poorest countries. With the latest deal, the total number of vaccine doses to be supplied to the U.S. government for donation by Pfizer/BioNTech adds up to 1 billion.
J&J’s Promising COVID-19 Booster Data: J&J presented additional data from the phase III ENSEMBLE study, which showed that a booster dose given 56 days after the first jab led to 94% protection against mild to severe COVID-19 in the United States. The data also demonstrated that when the booster jab was given two months after the first shot, antibody levels were four to six times higher than observed after the first vaccination. Also, antibody levels increased nine-fold one week after the booster dose, when the same was given six months after the first shot. J&J also said that the phase III data together with real-world evidence confirmed its vaccine’s strong and long-lasting protection against COVID-19-related hospitalizations and death, even after a single shot.
AstraZeneca’s Key Cancer Data at ESMO: AstraZeneca AZN presented detailed data from the head-to-head DESTINY-Breast03 phase III study on Enhertu at the European Society for Medical Oncology (ESMO) Congress. The data showed that Enhertu reduced the risk of death or tumor progression by 72% in patients with HER2-positive metastatic breast cancer previously treated with Roche’s Kadcyla (trastuzumab emtansine (T-DM1)) and a taxane. At ESMO, AstraZeneca also presented three-year data from the CASPIAN phase III study on its PD-L1 inhibitor, Imfinzi. The data showed that Imfinzi plus chemotherapy tripled patient survival at three years in first-line extensive-stage small cell lung cancer.
AstraZeneca signed a new collaboration with VaxEquity, an Imperial College London spin-off, to discover and develop self-amplifying next generation RNA therapeutics leveraging VaxEquity’s saRNA platform.
AbbVie Seeks Approval for Skyrizi in Crohn’s Disease: AbbVie ABBV submitted regulatory application in the United States seeking approval for its new drug Skyrizi (risankizumab) as a potential treatment for moderate to severe Crohn’s disease (“CD”) both as a 600mg intravenous (“IV”) induction and 360mg subcutaneous (“SC”) maintenance therapy. The filing is supported by data from three phase III studies – ADVANCE, MOTIVATE and FORTIFY – which evaluated the safety and efficacy of Skyrizi in CD patients. Skyrizi is presently approved to treat moderate to severe plaque psoriasis.
EU Signs Joint Procurement Deal for Lilly’s COVID-19 Cocktail Drug: Eli Lilly LLY signed a joint procurement agreement with the European Commission to supply 220,000 doses of its cocktail antibody medicine, bamlanivimab plus etesevimab together, for treating confirmed COVID-19. The agreement will allow participating European countries to purchase the cocktail drug directly from Lilly once the medicine is authorized by European regulatory authorities. The cocktail medicine is under review by the European Medicines Agency (“EMA”). In March, the EMA’s Committee for Medicinal Products for Human Use (“CHMP”) had given a position opinion recommending approval of bamlanivimab and etesevimab together as a treatment for COVID-19 in patients aged 12 and older.
Novartis’ New Acquisition: Novartis NVS acquired Arctos Medical, which expanded its optogenetics portfolio by adding the latter’s pre-clinical optogenetics-based AAV gene therapy program. The acquisition is in line with Novartis’ goal to make optogenetics-based therapies to restore vision to patients with advanced blindness.
Novartis announced a collaboration with SOLTI Innovative Cancer Research (SOLTI) to conduct HARMONIA study. HARMONIA is a phase III, head-to-head study evaluating Kisqali versus Pfizer’s Ibrance in patients with HR+/HER2- advanced breast cancer. The study aims to identify the best drug between Kisqali and Ibrance for patients with aggressive HER2-enriched intrinsic subtype of HR+/HER2- advanced breast cancer (ABC). Enrolment in the study is expected to begin in the first quarter of 2022.
The NYSE ARCA Pharmaceutical Index rose 0.2% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
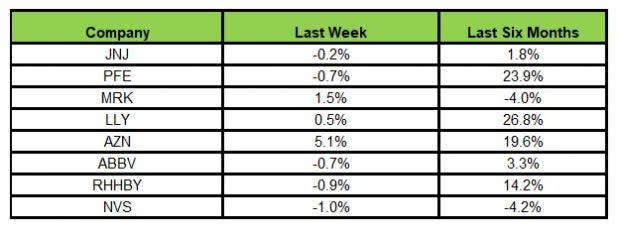
Image Source: Zacks Investment Research
In the last five trading sessions, AstraZeneca gained the highest (5.1%) while Novartis declined the most (1.0%)
In the past six months, Lilly recorded the maximum gain (26.8%) while Novartis declined the most (4.2%)
(See the last pharma stock roundup here: ABBV’s Deal With RGNX, LLY COVID Drug’s Government Order Win)
What's Next in the Pharma World?
Watch out for regular pipeline and regulatory updates next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 