Pharma Stock Roundup: PFE DMD Study Patient Death, FDA Panel Meet for LLY's Donanemab
This week, Pfizer PFE reported the death of a young boy who participated in a mid-stage study of its Duchenne muscular dystrophy (“DMD”) drug, fordadistrogene movaparvovec. An FDA advisory committee meeting to discuss Eli Lilly’s LLY Alzheimer’s candidate, donanemab, will be conducted on Jun 10. Merck’s MRK phase III study evaluating a Keytruda-based regimen for an endometrial cancer indication failed to meet the primary endpoint.
Recap of the Week’s Most Important Stories
Patient Death in Pfizer’s Phase II DMD Study: Pfizer issued a community letter informing of the death of a patient who participated in its ongoing phase II study, called DAYLIGHT, evaluating gene therapy fordadistrogene movaparvovec for treating DMD in boys aged 2-3 years. The boy received the gene therapy a year back in early 2023.
Pfizer said it did not have complete information about whether the death was caused due to the drug and is working on learning more details. It is also reviewing the data with the Data Monitoring Committee. Pfizer has paused dosing in the crossover arm of another ongoing phase III study called CIFFREO which is investigating fordadistrogene movaparvovec in patients 4-8 years of age with DMD. The pause does not apply to other ongoing studies in the DMD program, as dosing has already been completed.
FDA Panel Meeting to Discuss Lilly’s Donanemab BLA in June: The FDA announced that an in-person meeting of the Peripheral and Central Nervous System Drugs Advisory Committee (PCNS) will be convened on Jun 10 to discuss Lilly’s Alzheimer’s disease drug, donanemab.
Donanemab’s biologics license application (BLA) was based on data from the TRAILBLAZER-ALZ 2 phase III study, which evaluated the candidate in participants aged 60-85 years with early symptomatic Alzheimer's disease. Lilly had filed the BLA around mid-2023 and the FDA decision on donanemab was initially expected in the first quarter of 2024. However, in March 2024, the FDA said that a meeting of an advisory committee would be convened to discuss data from the TRAILBLAZER-ALZ 2 study to get more information about donanemab’s safety and effectiveness.
Data released last year from the TRAILBLAZER-ALZ 2 study showed that donanemab significantly slowed cognitive and functional decline in early symptomatic Alzheimer's disease patients.
Merck’s Keytruda Endometrial Cancer Study Fails: Merck’s phase III first-line endometrial cancer study on Keytruda failed to meet its primary endpoint of disease-free survival (DFS) at an interim analysis conducted by an independent Data Monitoring Committee. The study called KEYNOTE-B21 evaluated Keytruda in combination with chemotherapy as adjuvant treatment, with or without radiotherapy, for the treatment of patients with newly diagnosed, high-risk endometrial cancer after surgery with curative intent. As the study failed to reach superiority for the DFS endpoint, the study’s other primary endpoint of overall survival was not formally tested. Keytruda is presently approved for two approved indications in advanced endometrial carcinoma.
The NYSE ARCA Pharmaceutical Index rose 1.7% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
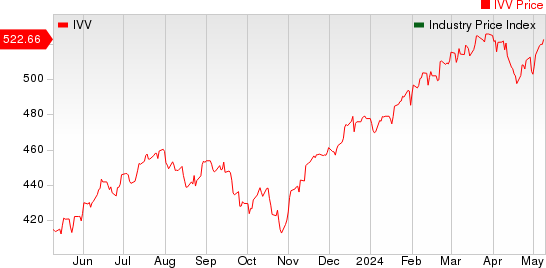
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
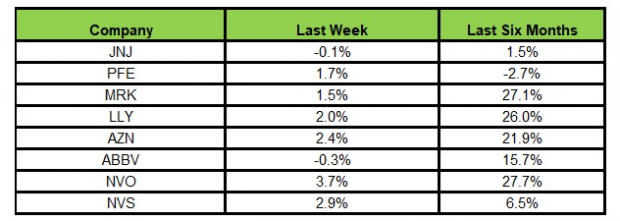
Image Source: Zacks Investment Research
In the last five trading sessions, Novo Nordisk rose the most (3.7%), while AbbVie declined the most (0.3%).
In the past six months, Novo Nordisk has risen the highest (27.7%), while Pfizer has declined the most (2.7%).
(See the last pharma stock roundup here: LLY, NVO, PFE Q1 Results, JNJ’s New Plan to Resolve Talc Claims)
What's Next in the Pharma World?
Watch this space for regular pipeline and regulatory updates next week.
Lilly, Pfizer and Merck have a Zacks Rank #3 (Hold) each. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 