Regeneron (REGN) Outperforms Industry YTD: What Lies Ahead?
Biotech giant Regeneron Pharmaceuticals REGN is one of the few top biotech companies that has outperformed the industry year to date.
Shares of the company have risen 19% year to date against the industry’s decline of 6.4%.
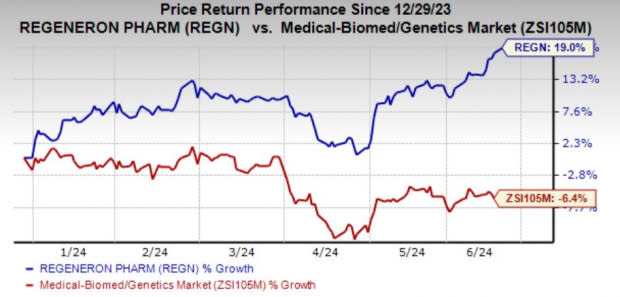
Image Source: Zacks Investment Research
Regeneron’s efforts to diversify its portfolio have impressed its investors, even as lead drug Eylea faces challenges.
Regeneron’s top line is being boosted by its share of profits/losses in connection with the global sales of Dupixent. Partner Sanofi SNY records global net product sales of Dupixent.
Solid sales of Dupixent (approved for use in certain patients with atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis and eosinophilic esophagitis) have fueled the top line.
Sanofi and Regeneron are striving hard to expand the drug’s label further. The European Medicines Agency’s Committee for Medicinal Products for Human Use recently adopted a positive opinion recommending the approval of Dupixent as an add-on maintenance treatment in adults with uncontrolled chronic obstructive pulmonary disease (COPD) characterized by raised blood eosinophils.
If approved, Dupixent would be the first-ever targeted therapy for COPD in the EU.
However, the FDA has extended its review period for a regulatory filing seeking label expansion of Dupixent in the COPD indication by an additional three months. A final decision is now expected by Sep 27, 2024. The regulatory body has requested additional analyses on the efficacy of Dupixent in the BOREAS and NOTUS pivotal trials.
Nonetheless, an approval for Dupixent in COPD indication should further boost this blockbuster drug’s sales, given the market potential.
Regeneron also has other arrows in its quiver. The company is looking to strengthen its oncology franchise, which currently comprises Libtayo (cemiplimab-rwlc), indicated in certain patients with advanced basal cell carcinoma, advanced cutaneous squamous cell carcinoma and advanced non-small cell lung cancer.
The FDA accepted the company’s biologics license application (BLA) seeking accelerated approval for linvoseltamab, a bispecific antibody targeting BCMA and CD3, to treat adult patients with relapsed/refractory (R/R) multiple myeloma that has progressed after at least three prior therapies.
The BLA was granted priority review with a target action date of August 22, 2024. A regulatory application is also under review in the EU.
The FDA, however, issued complete response letters (CRLs) for its BLA for odronextamab. The BLA is seeking the approval of the candidate in R/R follicular lymphoma (FL) and R/R diffuse large B-cell lymphoma. Regeneron stated that the sole issue with its approvability is related to the enrollment status of the confirmatory trials.
Earlier, REGN initiated a phase II/II study of the combination of fianlimab, an antibody to LAG-3, and Libtayo in first-line metastatic melanoma.
The successful development of these oncology drugs should be a great boost for REGN.
Lead drug Eylea is an anti-vascular endothelial growth factor inhibitor that is approved for various ophthalmology indications.
Eylea sales have been under pressure due to competition from Roche’s RHHBY Vabysmo. The uptake of Roche’s Vabysmo has been outstanding. Roche has designed Vabysmo to block pathways involving Ang-2 and VEGF-A.
To counter the decline in Eylea sales, Regeneron developed a higher dose of the drug. The initial uptake of Eylea HD is encouraging as Eylea patients transition to the higher dose.
Regeneron has a collaboration agreement with Bayer BAYRY for Eylea. Regeneron records the net product sales of Eylea and Eylea HD in the United States and Bayer records its net product sales outside the country. Regeneron records its share of profits in connection with sales of Eylea outside the United States.
While the near-term pipeline setbacks and challenges with Eylea might be a headwind, Regeneron has solid long-term growth prospects and should maintain the momentum going forward.
Zacks Rank
Regeneron currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Sanofi (SNY) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Bayer Aktiengesellschaft (BAYRY) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 