Regeneron's (REGN) Evkeeza Positive in Late-Stage Study
Regeneron Pharmaceuticals, Inc. REGN has announced positive results from a phase III study on Evkeeza (evinacumab)
The study was evaluating Evkeeza in children aged 5 to 11 with homozygous familial hypercholesterolemia (HoFH).
During the 24-week treatment period, patients received Evkeeza 15 mg/kg every four weeks via IV alongside their lipid-lowering treatment regimen. The primary endpoint was a change in LDL-C at week 24. Secondary endpoints included the effect of Evkeeza on other lipid parameters, efficacy by mutation status, safety and tolerability, immunogenicity and pharmacokinetics (PK).
Part A was a phase Ib study designed to assess the PK, safety and tolerability of Evkeeza. Patients who completed Part A or B were allowed to continue treatment in Part C, an ongoing phase III extension trial. Parts A, B and C were not designed to evaluate the effect of Evkeeza on cardiovascular events.
The study met its primary endpoint. Results showed that children who added investigational Evkeeza to other lipid-lowering therapies reduced their low-density lipoprotein-cholesterol (LDL-C) by 48% at week 24 on average. Children already on other lipid-lowering therapies entered the trial with dangerously high LDL-C (264 mg/dL on average), and 79% saw their LDL-C reduced by at least half at 24 weeks.
It is approved by the FDA (as evinacumab-dgnb) and the European Commission as an adjunct therapy for certain patients aged 12 years and older with HoFH.
Please note that Regeneron is responsible for the development and distribution of Evkeeza in the United States. It has collaborated with Ultragenyx (RARE) to clinically develop, commercialize and distribute Evkeeza outside of the United States.
Regeneron and Ultragenyx both collaborated for Evkeeza in January 2022.
REGN’s shares have gained 7.6% in the year so far against the industry’s decline of 23.7%.
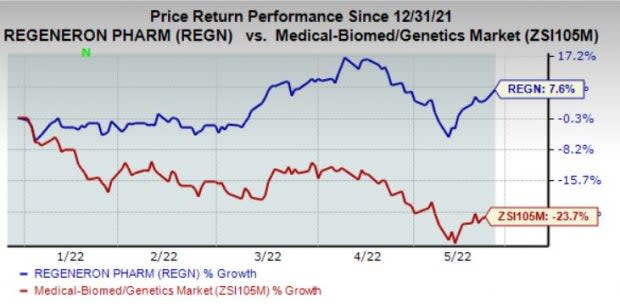
Image Source: Zacks Investment Research
Regeneron’s first-quarter results were strong with broad-based growth. Solid demand for Eylea and Dupixent maintained momentum for the company. Demand for lead ophthalmology drug Eylea (approved for neovascular age-related macular degeneration, diabetic macular edema and macular edema, among others) maintains momentum for the company on strong demand. Regeneron has a collaboration agreement with Bayer BAYRY for Eylea.
Recent label expansions into additional indications have boosted sales and should maintain momentum for the drug despite lingering competition. The company is working on expanding the drug’s label further, which should boost performance.
Regeneron records net product sales of Eylea in the United States. Bayer records net product sales of Eylea outside the United States.
However, sales from REGEN-COV took a hit due to the regulatory update.
Growth in Eylea and Dupixent through further penetration in existing indications and a promising late-stage pipeline set the momentum for growth. The approval of Libtayo in the lucrative indication of NSCLC should further boost the drug's sales in the upcoming quarters.
Regeneron currently carries a Zacks Rank #3 (Hold). A couple of better-ranked stocks are Alkermes ALKS and Geron Corporation GERN. While Alkermes sports a Zacks Rank #1 (Strong Buy), Geron has a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Loss estimates for ALKS for 2022 have narrowed to 3 cents from a loss of 14 cents in the past 60 days. Alkermes surpassed estimates in all of the trailing four quarters, the average surprise being 350.48%.
Loss estimates for GERN for 2022 have narrowed by 6 cents in the past 60 days. Geron surpassed estimates in three of the trailing four quarters, the average surprise being 1.07%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Geron Corporation (GERN) : Free Stock Analysis Report
Alkermes plc (ALKS) : Free Stock Analysis Report
Bayer Aktiengesellschaft (BAYRY) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 