Remdesivir becomes first coronavirus drug to get FDA approval
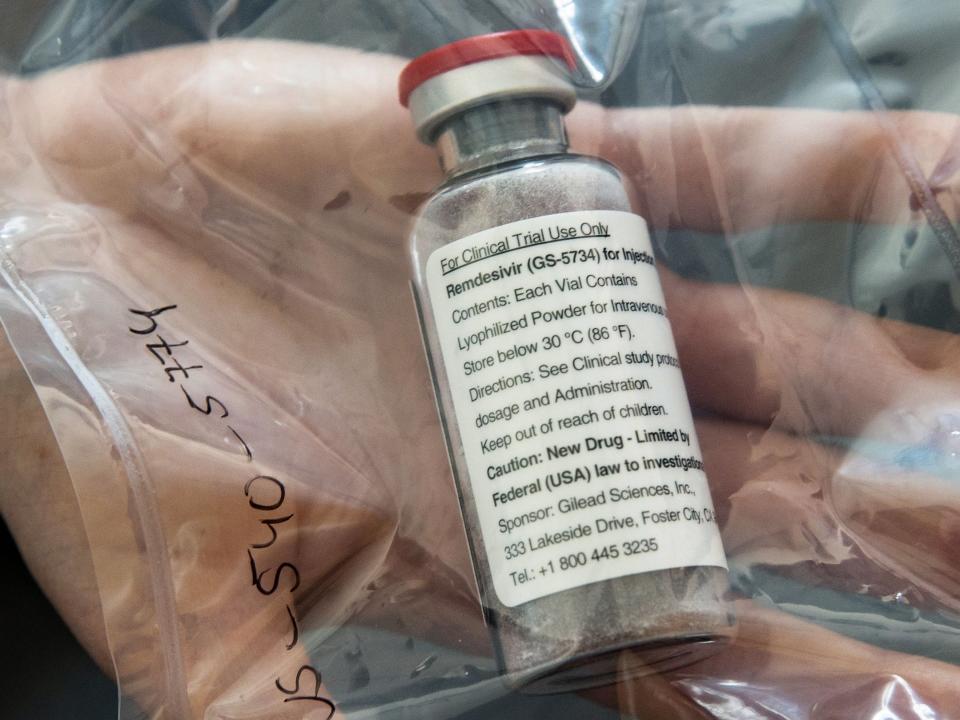
Remdesivir is the first antiviral medicine to receive approval from the Food and Drug Administration (FDA).
US regulators signed off on Thursday on the drug, which is given to hospitalised patients through an IV.
The drug has been authorized to be used in case of emergency since early in the pandemic but is the first treatment to win full approval from the FDA.
President Trump received the treatment after he contracted Covid-19 earlier this month and was hospitalized at Walter Reed Medical Center in Bethesda, Maryland.
The drug has been named Veklury by creators, the California-based Gilead Sciences. The treatment has cut recovery time by five days - from 15 days to 10 on average - according to a large study led by the National Institutes of Health.
Read more
‘Dramatic’ surge in Covid forces hospital to cancel operations

 Yahoo Finance
Yahoo Finance 