Sanofi's (SNY) Dupixent Gets FDA Nod for Prurigo Nodularis
Sanofi SNY and its partner Regeneron REGN recently announced that the FDA has approved their blockbuster medicine Dupixent (dupilumab) for the treatment of adult patients with prurigo nodularis, a chronic inflammatory skin disease.
Following the FDA nod, Dupixent became the first and only treatment to be approved for prurigo nodularis in the United States.
The latest FDA approval was based on data from two phase III studies, PRIME and PRIME2, which evaluated the safety and efficacy of Dupixent in adults with prurigo nodularis.
In May 2022, the FDA accepted Sanofi and Regeneron’s supplemental Biologics License Application (BLA) seeking label expansion of Dupixent for treating adult patients with prurigo nodularis. The FDA also granted priority review to the sBLA.
Shares of Sanofi have declined 23.8% so far this year compared with the industry’s decrease of 6.7%.
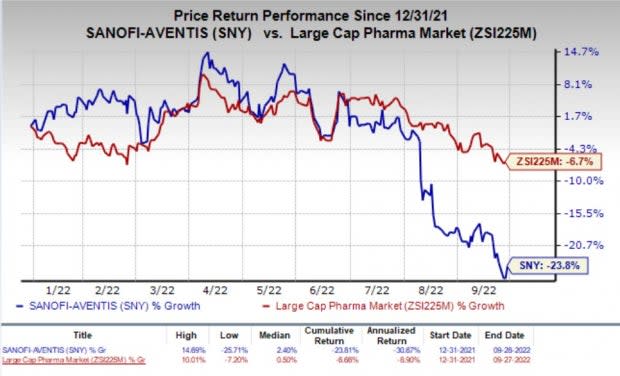
Image Source: Zacks Investment Research
Prurigo nodularis is now the fifth FDA-approved disease indication for Dupixent in the United States. Dupixent is already approved in the United States for several other type II inflammatory diseases, for certain patients with chronic rhinosinusitis with nasal polyposis, asthma, eosinophilic esophagitis and atopic dermatitis in different age populations.
Dupixent is being jointly marketed by REGN and SNY under a global collaboration agreement. Sanofi records global net product sales of Dupixent while Regeneron records its share of profits/losses in connection with the global sales of the drug.
Dupixent’s frequent label expansion approvals are driving sales further.
Sanofi and Regeneron are also studying dupilumab in late-stage studies for a broad range of diseases, driven by type II inflammation like pediatric eosinophilic esophagitis, chronic obstructive pulmonary disease, urticaria and bullous pemphigoid.
Zacks Rank & Other Stocks to Consider
Sanofi currently carries a Zacks Rank #2 (Buy). Other stocks worth considering in the biotech sector include Aptose Biosciences Inc. APTO and Atara Biotherapeutics, Inc. ATRA, both carrying Zacks Rank #2 at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Aptose Biosciences’ loss per share estimates narrowed by 15.7% for 2022 and 15% for 2023 in the past 60 days.
Earnings of Aptose Biosciences surpassed estimates in three of the trailing four quarters and missed on the remaining occasion. APTO delivered an earnings surprise of 2.23%, on average.
Atara Biotherapeutics’ loss per share estimates narrowed 43.2% for 2022 and 21.3% for 2023 in the past 60 days.
Earnings of Atara Biotherapeutics surpassed estimates in three of the trailing four quarters and missed on the other occasion. ATRA delivered an earnings surprise of 4.83%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Sanofi (SNY) : Free Stock Analysis Report
Atara Biotherapeutics, Inc. (ATRA) : Free Stock Analysis Report
Aptose Biosciences, Inc. (APTO) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 