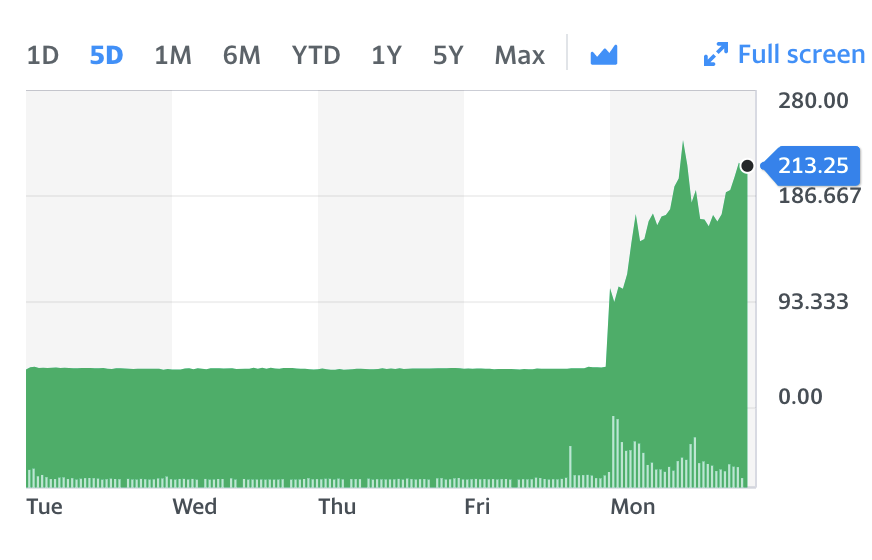
Shares in Southampton firm surge 450% on ‘breakthrough’ coronavirus treatment

Shares in Southampton-based Synairgen (SNG.L) climbed by more than 450% on Monday after the biotechnology firm reported positive results from a trial of an experimental treatment for hospitalised coronavirus patients.
In a statement, the company said that the treatment, known as SNG001, “greatly reduced” the number of COVID-19 patients who needed to go on a ventilator.
The odds of developing severe disease during a 16-day treatment period were reduced by 79% for patients receiving the treatment compared to those who received a placebo, it said.
In an interview with the BBC, Synairgen chief executive Richard Marsen hailed the results, describing them as “a major breakthrough in the treatment of hospitalised COVID-19 patients.”
“We couldn't have expected much better results than these,” he said.
READ MORE: Stocks rise on 'promising' vaccine trial and EU summit hopes
The treatment sees patients inhale a protein called interferon beta directly into their lungs using a nebuliser, a procedure designed to stimulate an immune response to COVID-19.
Synairgen said that, compared with those on the placebo, patients who received SNG001 were more than twice as likely to recover from the illness to such an extent that everyday activities were not compromised.
Over the treatment period, the measure of breathlessness was “markedly reduced,” it said.
While the treatment appears to prevent the conditions of those with moderate illness from worsening, the study did not produce any statistically significant evidence that those who were admitted to hospital with severe cases of coronavirus were more likely to be discharged from hospital.
READ MORE: GSK invests £130m in Germany’s CureVac
The results from the Synairgen study come after a paper in a leading medical journal said a potential vaccine in a University of Oxford trial had been found safe.
“These early results hold promise,” said Professor Sarah Gilbert of the University of Oxford.
“If our vaccine is effective, it is a promising option as these types of vaccine can be manufactured at large scale.”
AstraZeneca has already signed deals with the university and with countries including the UK and the US to supply doses of the vaccine if trials are successful.

 Yahoo Finance
Yahoo Finance 
