Spectrum Pharmaceuticals (SPPI) Gets Nod for Rolvedon Injection
Spectrum Pharmaceuticals' SPPI shares increased 8.11% after market hours on Sep 9, after the company announced that it received FDA approval for its drug candidate, eflapergrastim-xnst, under the trade name Rolvedon injection.
The company announced that eflapergrastim has been approved by the FDA for the indication to reduce infection caused by febrile neutropenia in adults with non-myeloid malignancies receiving myelosuppressive chemotherapy.
The company first submitted a biologics license application (BLA) with the FDA for eflapergrastim in December 2018. The BLA was based on the data from two phase III clinical studies — ADVANCE and RECOVER— evaluating the safety and efficacy of the drug in early-stage breast cancer patients for treating neutropenia due to myelosuppressive chemotherapy.
However, Spectrum voluntarily withdrew its BLA for eflapergrastim in March 2019 after the FDA requested additional manufacturing-related information for the candidate.
Spectrum then submitted an updated BLA with the FDA for eflapergrastim for the same indication, based on the same data. It also included the manufacturing process data previously requested by the FDA.
In 2021, the company received a complete response letter (CRL) from the FDA regarding the BLA for eflapergrastim, indicating deficiencies related to manufacturing. FDA also indicated that a reinspection would be necessary.
After the completion of the remediation of these deficiencies, the company resubmitted the BLA in March, which the FDA accepted in April 2022.
The approval of Rolvedon (eflapergrastim) brings a robust opportunity for Spectrum to compete in the $2-billion target market. The approval also transforms Spectrum into a commercial-stage company.
Spectrum has already started the commercialization of the drug and intends to make it available in the U.S market in the fourth quarter of 2022.
Shares of Spectrum have returned 2% in the year-to-date period, against the industry’s decline of 21.1%.
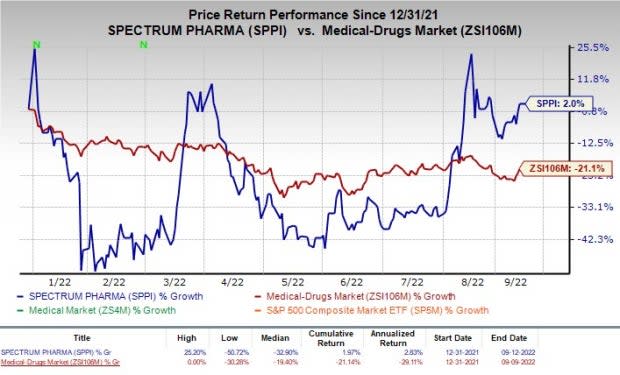
Image Source: Zacks Investment Research
The biopharma company has yet another late-stage candidate, poziotinib, in its portfolio.
Poziotinib is also an irreversible tyrosine kinase inhibitor being evaluated for Non-Small Cell Lung Cancer (NSCLC) tumors with various mutations.
The company already submitted a new drug application (NDA) to the FDA for the poziotinib back in December 2021 for use in patients with previously treated locally advanced or metastatic NSCLC with HER2 exon 20 insertion mutations.
The FDA accepted the NDA for poziotinib for NSCLC tumors with various mutations earlier in 2022, and its decision is expected on Nov 24, 2022. Poziotinib has also received Fast Track designation from the FDA. To date, no treatment has been approved by the FDA specifically for the aforementioned indication.
Spectrum Pharmaceuticals, Inc. Price
Spectrum Pharmaceuticals, Inc. price | Spectrum Pharmaceuticals, Inc. Quote
Zacks Rank and Stocks to Consider
Spectrum currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the same sector are Aerie Pharmaceuticals AERI, Catalyst Pharmaceuticals CPRX and Soleno Therapeutics SLNO,each carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 (Strong Buy) Rank stocks here.
Aerie’s loss per share estimates for 2022 have improved from $1.91 to $1.83 in the past 30 days. The same for 2023 has narrowed down to $1.01 from $1.05 in the same time frame.
Earnings of Aerie beat estimates in three of the trailing four quarters while missing the same in the remaining occasion. The average earnings surprise for AERI is 70.27%.
Catalyst’s earnings per share estimates for 2022 have improved from 67 cents to 70 cents in the past 30 days. The same for 2023 has improved from 79 cents to 85 cents in the same time frame.
Earnings of Catalyst missed estimates in two of the trailing four quarters, were in line in one and beat the same on the remaining occasion. The average negative earnings surprise for CPRX is 5.41%.
Soleno’s loss per share estimates for 2022 have narrowed down from $4.20 to $4.05 in the past 30 days. The same for 2023 has narrowed from $3.00 to $2.10 in the same time frame.
Earnings of Soleno missed estimates in three of the trailing four quarters and were in line on the remaining occasion. The average earnings surprise for SLNO is 11.40%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Spectrum Pharmaceuticals, Inc. (SPPI) : Free Stock Analysis Report
Catalyst Pharmaceuticals, Inc. (CPRX) : Free Stock Analysis Report
Aerie Pharmaceuticals, Inc. (AERI) : Free Stock Analysis Report
Soleno Therapeutics, Inc. (SLNO) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 