Spero (SPRO) Application for UTI Drug Gets Priority Review
Spero Therapeutics, Inc. SPRO announced that the FDA granted Priority Review designation to its new drug application (NDA) for tebipenem HBr oral tablets.
The NDA seeks approval of the candidate for treatment in adult patients with complicated urinary tract infections (cUTI), including acute pyelonephritis, caused by susceptible microorganisms. Tebipenem HBr has previously been granted Qualified Infectious Disease Product (QIDP) and Fast Track designations for these cUTI indications.
The regulatory body is planning to hold an Advisory Committee meeting to discuss this NDA and set a target action date of Jun 27, 2022.
The NDA submission includes previously-reported positive data from the phase III ADAPT-PO study, which showed that ADAPT-PO met its primary endpoint by demonstrating that oral tebipenem HBr was statistically non-inferior to intravenous (IV) ertapenem in the treatment of patients with cUTI and patients with acute pyelonephritis (AP).
Shares of the company have lost 9.1% in the past year compared with the industry’s decline of 21.9%.
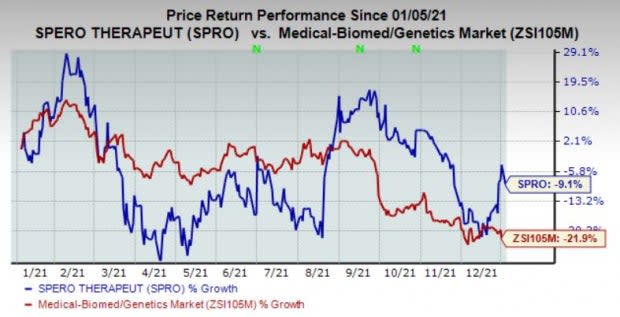
Image Source: Zacks Investment Research
Tebipenem HBr is Spero’s lead product candidate and is being developed as the first oral carbapenem antibiotic for use in cUTI, including pyelonephritis.
The successful development of the candidate will be a significant boost for this clinical-stage biopharmaceutical company.
Spero is also developing SPR720 as a novel oral therapy product candidate for the treatment of a rare, orphan pulmonary disease caused by non-tuberculous mycobacterial infections.
The company has an IV-administered next-generation polymyxin product candidate, SPR206. The candidate has been developed from its potentiator platform to treat MDR Gram-negative infections in the hospital setting.
The development of SPR206 is supported by funding from the National Institute of Allergy and Infectious Diseases and is subject to a license agreement with Pfizer Inc., PFE.
Pfizer made a $40-million equity investment in Spero. Pursuant to this agreement between the parties, Spero granted Pfizer the rights to develop, manufacture and commercialize SPR206 in ex-U.S. and ex-Asia territories. In exchange, Spero is eligible to receive up to $80 million in development and sales milestones and high single-digit to low double-digit royalties on net sales of SPR206 in these territories.
Spero currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the healthcare sector include GlaxoSmithKline GSK and Gilead Sciences, Inc. GILD, each carrying a Zacks Rank of 2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
GlaxoSmithKline’s estimates for 2022 earnings per share have moved north from $2.94 to $3.05 in the past 60 days. GSK has increased 16.9% in the year.
Earnings of GlaxoSmithKline beat estimates in three of the last four quarters and missed the mark once. It has a trailing four-quarter earnings surprise of 15.3%, on average.
Estimates for Gilead have increased to $6.75 from $6.72 for 2022 in the past 30 days. GILD has increased 20.7% in the year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
Spero Therapeutics, Inc. (SPRO) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 