Trevena Announces FDA Acceptance of Olinvo NDA, Shares Up
Shares of Trevena, Inc. TRVN rallied almost 24.5% in after-market trading on Jan 2, following the company’s announcement that the FDA has accepted the new drug application (“NDA”) for its pain therapy, Olinvo injection. Olinvo is an opioid-based analgesic administered intravenous and also the first G protein biased ligand of the mu receptor.
The company expects a decision from the FDA in the fourth quarter of 2018.
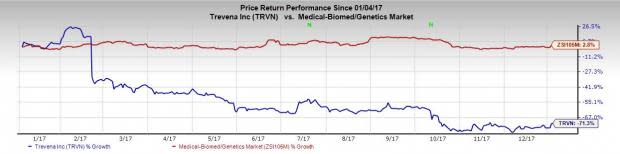
Shares of Trevena have lost 71.3% in the past year, significantly underperforming the industry’s gain of 2.8%. A potential approval from the FDA can give a boost to the stock.
The NDA included data from three phase III studies – APOLLO-1, APOLLO-2 and ATHENA – and a phase II study, which evaluated Olinvo in patients with moderate-to-severe acute pain.
Data from phase II & ATHENA studies have shown that Olinvo has achieved rapid and powerful analgesic efficacy and a wider therapeutic window versus morphine. Data from APOLLO-1 and APOLLO-2 also showed that the drug achieved statistically greater analgesic efficacy compared to placebo.
Trevena does not have any approved product in its portfolio and did not record any revenues in the third quarter of 2017.
The company believes that upon a potential approval it will incur significant expenses for commercialization of the drug. The company will have to seek various ways to raise funds. Also, it will face competition from currently marketed drugs, which include AcelRx Pharmaceuticals, Inc.’s ACRX Dsuvia and Mallinckrodt Public Limited Company’s MNK Exalgo.
Meanwhile, the company is also developing two G protein biased ligand candidates — TRV250, as a non-narcotic treatment for migraine and TRV734 for moderate-to-severe acute and chronic pain.
Trevena, Inc. Price

Trevena, Inc. Price | Trevena, Inc. Quote
Zacks Rank & Stock to Consider
Trevena carries a Zacks Rank #3 (Hold).
Corcept Therapeutics Incorporated CORT is a better-ranked health care stock, carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Corcept’s earnings per share estimates have increased from 77 cents to 88 cents for 2018 over the last 60 days. The company delivered a positive earnings surprise in two of the trailing four quarters with an average beat of 14.32%. The company’s stock is up 146.4% so far this year.
Investor Alert: Breakthroughs Pending
A medical advance is now at the flashpoint between theory and realization. Billions of dollars in research have poured into it. Companies are already generating substantial revenue, and even more wondrous products are in the pipeline.
Cures for a variety of deadly diseases are in sight, and so are big potential profits for early investors. Zacks names 5 stocks to buy now.
Click here to see them >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Trevena, Inc. (TRVN) : Free Stock Analysis Report
AcelRx Pharmaceuticals, Inc. (ACRX) : Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
Mallinckrodt PLC (MNK) : Free Stock Analysis Report
To read this article on Zacks.com click here.

 Yahoo Finance
Yahoo Finance 