Adeno-associated Virus Gene Therapy Market Report 2024-2034: Epidemiology, Industry Trends and Forecasts

Adeno-associated Virus Gene Therapy Market
Dublin, June 21, 2024 (GLOBE NEWSWIRE) -- The "Adeno-associated Virus Gene Therapy Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034" report has been added to ResearchAndMarkets.com's offering.
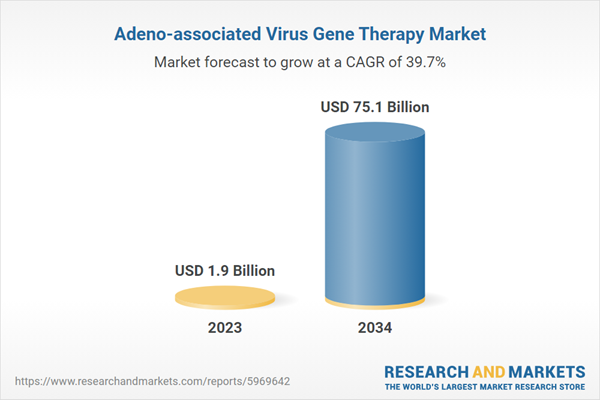
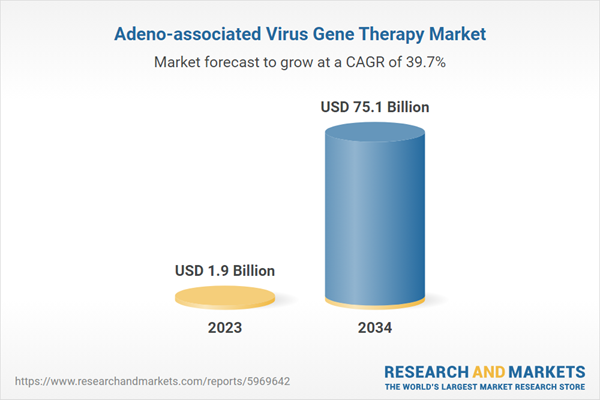
The 7 major adeno-associated virus gene therapy markets reached a value of US$ 1.9 Billion in 2023 and is forecast to reach US$ 75.1 Billion by 2034, exhibiting a growth rate (CAGR) of 50.46% during 2023-2034.
The increasing ability of AAV gene therapy to treat a broad array of disorders, including inherited diseases, certain types of cancer, and numerous viral infections, is primarily driving the global adeno-associated virus gene therapy market. In addition to this, the emerging popularity of the AAV vector owing to its several unique features that are beneficial in clinical applications, including low immunogenicity, broad tropism, and ease of production, is further creating a positive outlook for the market. AAV is also non-pathogenic, rarely integrates into the host chromosome, and results in long-term expression of the transgene.
Moreover, various key players are extensively investing in AAV gene therapy research and development activities for the high-yield producer cell lines, which would enable improved quality and more flexible viral-vector production at a lower cost. This, in turn, is further acting as a significant growth-inducing factor. Additionally, the escalating utilization of AAV gene therapy for personalized treatment since it can be easily tailored to the patient's specific genetic mutation, leading to more effective treatment, is also bolstering the global market. Furthermore, continuous advancements in the biotechnological and genetics sectors, such as modifying AAV transduction efficiency by optimizing the transgene cassette and utilizing capsid engineering to increase vector tropism, are expected to drive the global adeno-associated virus gene therapy market in the coming years.
This report provides an analysis of the adeno-associated virus gene therapy market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc.
The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for adeno-associated virus gene therapy and also represents the largest market for its treatment.
Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the adeno-associated virus gene therapy market in any manner.
Time Period of the Study
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
United States
Germany
France
United Kingdom
Italy
Spain
Japan
Analysis Covered Across Each Country
Historical, current, and future epidemiology scenario
Historical, current, and future performance of the adeno-associated virus gene therapy market
Historical, current, and future performance of various therapeutic categories in the market
Sales of various drugs across the adeno-associated virus gene therapy market
Competitive Landscape:
This report also provides a detailed analysis of the current adeno-associated virus gene therapy marketed drugs and late-stage pipeline drugs.
In-Market Drugs
Drug overview
Mechanism of action
Regulatory status
Clinical trial results
Drug uptake and market performance
Late-Stage Pipeline Drugs
Drug overview
Mechanism of action
Regulatory status
Clinical trial results
Drug uptake and market performance
Key Attributes:
Report Attribute | Details |
No. of Pages | 129 |
Forecast Period | 2023 - 2034 |
Estimated Market Value (USD) in 2023 | $1.9 Billion |
Forecasted Market Value (USD) by 2034 | $75.1 Billion |
Compound Annual Growth Rate | 39.7% |
Regions Covered | Global |
Key Questions Answered in this Report:
Market Insights
How has the adeno-associated virus gene therapy market performed so far and how will it perform in the coming years?
What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
What was the country-wise size of the adeno-associated virus gene therapy market across the seven major markets in 2023 and what will it look like in 2034?
What is the growth rate of the adeno-associated virus gene therapy market across the seven major markets and what will be the expected growth over the next ten years?
What are the key unmet needs in the market?
Epidemiology Insights
What is the number of cases (2018-2034) going for adeno-associated virus gene therapy across the seven major markets?
What is the number of cases (2018-2034) going for adeno-associated virus gene therapy by age across the seven major markets?
What is the number of cases (2018-2034) going for adeno-associated virus gene therapy by gender across the seven major markets?
What is the number of cases (2018-2034) going for adeno-associated virus gene therapy by type across the seven major markets?
How many patients are diagnosed (2018-2034) with adeno-associated virus gene therapy across the seven major markets?
What is the size of the adeno-associated virus gene therapy patient pool (2018-2023) across the seven major markets?
What would be the forecasted patient pool (2024-2034) across the seven major markets?
What are the key factors driving the epidemiological trend of adeno-associated virus gene therapy?
What will be the growth rate of patients across the seven major markets?
Adeno-associated Virus Gene Therapy: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
What are the current marketed drugs and what are their market performance?
What are the key pipeline drugs and how are they expected to perform in the coming years?
How safe are the current marketed drugs and what are their efficacies?
How safe are the late-stage pipeline drugs and what are their efficacies?
What are the current treatment guidelines for adeno-associated virus gene therapy drugs across the seven major markets?
Who are the key companies in the market and what are their market shares?
What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the adeno-associated virus gene therapy market?
What are the key regulatory events related to the adeno-associated virus gene therapy market?
What is the structure of clinical trial landscape by status related to the adeno-associated virus gene therapy market?
What is the structure of clinical trial landscape by phase related to the adeno-associated virus gene therapy market?
What is the structure of clinical trial landscape by route of administration related to the adeno-associated virus gene therapy market?
For more information about this report visit https://www.researchandmarkets.com/r/gchwtb
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
CONTACT: CONTACT: ResearchAndMarkets.com Laura Wood,Senior Press Manager press@researchandmarkets.com For E.S.T Office Hours Call 1-917-300-0470 For U.S./ CAN Toll Free Call 1-800-526-8630 For GMT Office Hours Call +353-1-416-8900


 Yahoo Finance
Yahoo Finance 
