Amgen's Migraine Drug Aimovig Gets FDA Approval, Shares Up
Amgen AMGN and partner Novartis NVS announced that the FDA has approved their pipeline candidate Aimovig (erenumab) for the prevention of migraine.
The drug is the first FDA-approved treatment, specifically developed to treat migraine by blocking calcitonin gene-related peptide ("CGRP") receptor. Aimovig will be available in a pre-filled self-administering autoinjector, SureClick, for a once monthly 70 mg dosage. Aimovig is under review in the EU.
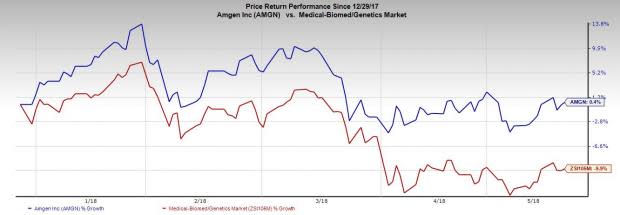
Amgen’s shares were up 1.3% in after-hours trading following the announcement. Year to date, Amgen’s shares have increased 0.4% compared with the industry’s decline of 9.9%.
Aimovig’s approval is backed by positive data from several mid and late stage studies. The drug significantly reduced monthly migraine days and use of acute migraine medications versus placebo in studies. Data from a phase IIIb LIBERTY study in episodic migraine patients who failed two to four prior treatments showed that Aimovig has three times higher probability of reducing migraine days by half compared to placebo.
New treatment options, specifically developed to treat migraine, are making their way, suggesting potential demand for Aimovig. Amgen has priced the drug at $575 for a once monthly 70 or 140 mg single-use prefilled SureClick autoinjector or $6,900 annually. We expect the drug to boost Amgen’s top line going forward.
We remind investors that Eli Lilly LLY and Teva Pharmaceutical Industries Limited TEVA also have anti-CGRP candidates, galcanezumab and fremanezumab, respectively, which are under review in the United States.
A decision on galcanezumab is expected in the third quarter of the year
A FDA decision on fremanezumab was expected in June. However, earlier this month, Teva informed that it does not expect to get FDA approval for fremanezumab on its PDUFA date in June. This is because Teva’s manufacturing partner for fremanezumab, Celltrion, which makes the API, received a warning letter from the FDA this year that delayed the approval of the drug. However, Teva expects fremanezumab to be approved and launched before the end of this year.
Amgen Inc. Price

Amgen Inc. Price | Amgen Inc. Quote
Amgen currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Amgen Inc. (AMGN) : Free Stock Analysis Report
Teva Pharmaceutical Industries Ltd. (TEVA) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 
