FDA Expands Sarepta's (SRPT) DMD Gene Therapy Label
Shares of Sarepta SRPT rose nearly 34% in after-market trading on Thursday after it announced that the FDA approved the expanded use of its Duchenne muscular dystrophy (DMD) gene therapy Elevidys.
Elevidys is now approved to treat all DMD patients aged four years and older. While the FDA granted traditional approval for the therapy to treat ambulatory DMD patients (those who can still walk), it has granted accelerated approval for non-ambulatory patients.
Sarepta’s gene therapy was initially approved by the FDA last year under the accelerated pathway to treat ambulatory pediatric patients aged between four and five years with DMD. Following the FDA nod, Elevidys was the first-ever approved gene therapy for DMD.
The label expansion is mainly supported by data from the phase III EMBARK study, announced last October. Though the study failed to achieve its primary endpoint, it achieved statistical significance on all pre-specified key secondary endpoints, indicating that treatment with Elevidys modifies the course of DMD indication.
Management will still need to conduct a confirmatory study to convert the accelerated approval for non-ambulatory DMD patients to a full one. Sarepta is currently conducting the phase III ENVISION study to evaluate the safety and efficacy of gene therapy in non-ambulatory and ambulatory DMD patients. This study also satisfies the regulatory requirements for Elevidys’ approval outside the United States.
Elevidys is the only one-shot gene therapy for DMD in the United States. A progressive and degenerative disorder, DMD leads to weakness and wasting away of the body’s muscles. Despite being initially approved with a confined label, Sarepta recorded revenues of more than $200 million from Elevidys sales last year, which is an encouraging figure for a therapy that was commercially launched in the second half of 2023. This uptick in share price, following the label expansion announcement, is likely attributed to Elevidys’ blockbuster potential.
Year to date, Sarepta’s shares have inched up 28.1% against the industry’s 7.9% fall.
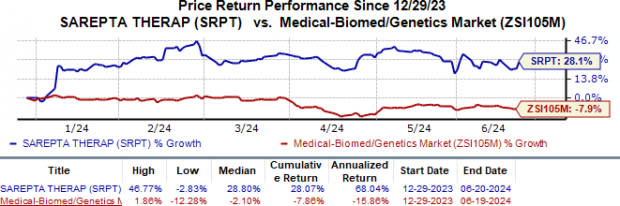
Image Source: Zacks Investment Research
Sarepta developed Elevidys in collaboration with Roche RHHBY. Sarepta and Roche entered into a licensing agreement in 2019 to develop and commercialize Elevidys. Per the agreement, Sarepta is responsible for marketing the therapy in the United States, while Roche is responsible for marketing the gene therapy outside the country. Sarepta is also eligible to receive collaboration revenues on the ex-U.S. sales made by Roche.
Sarepta is currently the market leader in DMD treatment. Apart from Elevidys, the company has three other therapies in its commercial portfolio targeting the DMD patient population, namely Exondys 51, Vyondys 53 and Amondys 45. Per management, these three drugs have the potential to address nearly a third of all patients with DMD in the United States.
However, competition in the DMD space is stiff as companies like Regenxbio RGNX and Solid Biosciences SLDB have been developing their respective gene therapy candidates for DMD.
Regenxbio is planning to hold an end-of-phase II (EOP2) meeting with the FDA early in the next quarter to discuss the study design of a pivotal late-stage study on RGX-202, its DMD gene therapy candidate. Based on the feedback, Regenxbio expects to start this pivotal study before 2024-end. If the outcome of this study is positive, it plans to seek accelerated approval for its therapy from the FDA.
Last year, the FDA cleared Solid Biosciences’ investigational new drug (IND) to start a phase I/II study on SGT-003 in pediatric patients with DMD. This study is expected to begin by the end of this month. SLDB expects to report initial data from this study by this year’s end. The FDA has granted fast-track, orphan drug and rare pediatric disease designations to Solid Biosciences’ gene therapy in DMD indication.
However, companies also have suffered major setbacks in the DMD space. Earlier this month, Pfizer PFE announced that a late-stage study evaluating its DMD gene therapy candidate failed to meet the primary endpoint and key secondary endpoints. Treatment with the Pfizer therapy failed to improve motor function among the DMD boys. Pfizer plans to share more detailed results from the study at an upcoming medical meeting.
Sarepta Therapeutics, Inc. Price
Sarepta Therapeutics, Inc. price | Sarepta Therapeutics, Inc. Quote
Zacks Rank
Sarepta sports a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Sarepta Therapeutics, Inc. (SRPT) : Free Stock Analysis Report
REGENXBIO Inc. (RGNX) : Free Stock Analysis Report
Solid Biosciences Inc. (SLDB) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 