Ligand (LGND) Posts Long-Term Outlook, Updates Portfolio Progress
At its R&D event held on Dec 12, Ligand Pharmaceuticals LGND provided an overview of its business model and issued fresh guidance outlook for the next five years.
Financial Guidance
For 2024
Ligand issued fresh guidance for 2024. Management expects total revenues in the range of $130-$142 million.
Royalty revenues are expected to be between $90 million and $95 million, while contract revenues are expected in the range of $15-$20 million. The company expects Captisol sales between $25-$27 million.
The company expects adjusted diluted earnings per share (EPS) between $4.25 and $4.75.
The guidance excludes Captisol sales related to COVID-19.
Five-Year Outlook
Ligand expects existing commercial programs and late-stage pipelines to provide a strong foundation for its business model. In this regard, management expects its royalty revenues to witness a compounded annual growth rate (CAGR) of 16% over the next five years. Management expects that by investing in new deals, the royalty revenues could exceed 20% CAGR growth.
By 2028, Ligand expects to record $290 million in total core revenues and achieve adjusted diluted EPS in the range of $10.00-$10.50. Based on these factors, it expects that the EBITDA margin could cross 80%.
Year to date, shares of Ligand have lost 5.8% compared with the industry’s 19.9% decline.
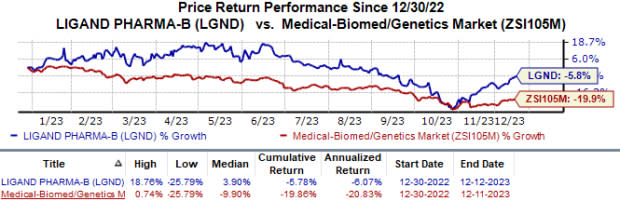
Image Source: Zacks Investment Research
Business Updates
Along with the guidance, Ligand also provided an update on its existing business model and reviewed the major pipeline events expected next year.
Recently, management has been seeking business opportunities aimed to help accelerate its profitability while lowering its infrastructure costs at the same time.
In September, Ligand acquired certain assets of Novan for $12.2 million. The assets include full ownership rights to berdazimer gel, which is under FDA review as a potential treatment for molluscum contagiosum infection. A final decision is expected before Jan 5, 2024.
In October, Ligand acquired Tolerance Therapeutics, the inventors of Sanofi-marketed Tzield, which is the only FDA-approved drug to delay the onset of stage 3 type 1 diabetes. It is eligible to receive royalties on global Tzield sales. During the same month, Ligand also acquired a 13% interest from Ovid Therapeutics OVID in the royalties and milestone payments of CH24H inhibitor soticlestat, which has shown the potential to reduce seizure susceptibility and improve seizure control. In return, Ovid Therapeutics will receive a $30-million payment, minus certain reimbursable expenses.
OVID sold its rights in soticlestat to Takeda in 2021. Takeda is currently developing the drug in pivotal late-stage studies for Lennox-Gastaut syndrome (LGS) and Dravet syndrome (DS) indications. Data from these studies are expected in 2024.
Ligand’s partner, Verona Pharma VRNA, also filed a new drug application (NDA) seeking approval for ensifentrine for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). A final decision on Verona’s NDA is expected in June. Per Verona, the FDA does not plan to hold an advisory committee meeting to discuss the NDA.
The company’s partner, Viking Therapeutics VKTX, is also expected to report 52-week biopsy results from the ongoing phase IIb VOYAGE study by first-half 2024. The VOYAGE study is evaluating Viking Therapeutics’ VK-2809 in non-alcoholic steatohepatitis (NASH) indication over 52 weeks.
After the spun-out of its Pelican subsidiary in September, Ligand is focused on maintaining and licensing its Captisol technology platform to generate royalties. In this regard, management expects regulatory approvals in the next year for five products (partnered with Eisai, BendarRx, SQ innovation, Sunshine Lake Pharma and Merck KGaA) that utilize the Captisol technology.
Ligand Pharmaceuticals Incorporated Price
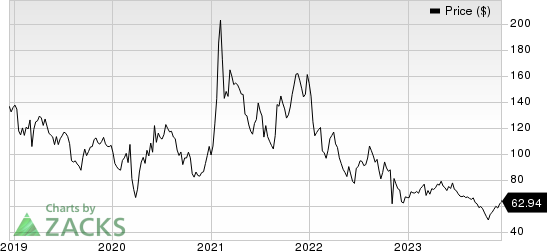
Ligand Pharmaceuticals Incorporated price | Ligand Pharmaceuticals Incorporated Quote
Zacks Rank
Ligand currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report
Viking Therapeutics, Inc. (VKTX) : Free Stock Analysis Report
Verona Pharma PLC American Depositary Share (VRNA) : Free Stock Analysis Report
Ovid Therapeutics (OVID) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 