Reata (RETA) Stock Jumps as FDA Approves Rare Disease Drug
Reata Pharmaceuticals RETA stock skyrocketed almost 200% on Wednesday, a day after the FDA approved its drug Skyclarys, or omaveloxolone, across a broad patient population of an ultra-rare disease called Friedreich’s ataxia.
Shares of Reata have surged 200.3% in the past year against the industry’s 9.9% decline.
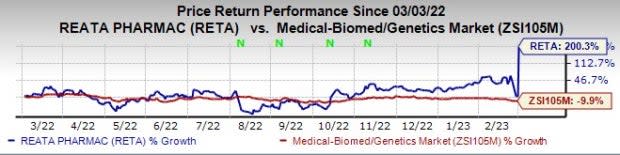
Image Source: Zacks Investment Research
The FDA approved Skyclarys for treating Friedreich’s ataxia in adults and adolescents aged 16 years and older.
Friedreich’s ataxia is an ultra-rare genetic, progressive, neurodegenerative movement disorder that affects approximately 5,000 diagnosed patients in the United States. This disease, which is usually diagnosed during adolescence, causes progressive loss of coordination, muscle weakness and fatigue, eventually resulting in loss of mobility and finally death.
The recommended dosage of Skyclarys is 150 mg taken orally once daily. The FDA actually gave a broad label to Skyclarys. Its label does not include any safety-related warning like a boxed warning or a drug safety program like Risk Evaluation and Mitigation Strategy. Only patients’ bilirubin levels and lipid parameters should be monitored prior to the initiation of Skyclarys. The FDA has also not issued any post-approval requirements. Reata has priced Skyclarys at an annual cost of $370,000. Reata expects patients to gain access through insurance and a patient-assistance program.
The FDA approval of Skyclarys was based on efficacy and safety data from the MOXIe Part 2 study and a post hoc analysis of the open-label MOXIe extension study. Data from the MOXIe Part 2 study showed that treatment with Skyclarys led to statistically significant lower impairment in patients with Friedreich’s ataxia compared to placebo at Week 48.
Skyclarys is the first drug to be approved specifically for the treatment of Friedreich’s ataxia and is also Reata’s first commercial product. There are no currently approved disease-modifying therapies for Friedreich’s ataxia, which means Reata will enjoy the first-mover advantage for some time. Skyclarys is expected to generate significant revenues for the company. Reata expects the drug to be available commercially in the second quarter of 2023.
Several analysts hiked their price targets on the stock after the FDA’s approval for Skyclarys.
In addition to the approval, the FDA also granted Reata a rare pediatric disease priority review voucher.
Reata also filed its 10-K report wherein it mentioned its financial results for 2022. Total revenues, comprising collaboration revenues, were $2.2 million in 2022 compared with $11.5 million in 2021. The company’s net loss was $311.9 million in 2023, wider than $297.4 million in 2021. R&D expenses were $169.8 million in 2022, up almost 9% year over year. SG&A expenses were $109.3 million, up 10.4% year over year.
Another company that is making a drug for Friedreich’s ataxia is PTC Therapeutics PTCT.
PTC Therapeutics is developing vatiquinone (PTC743) in phase III pivotal studies for children and young adults with Friedreich ataxia. PTC Therapeutics’ vatiquinone has been granted Orphan Drug Designation and Fast Track Designation by the FDA. PTC Therapeutics expects to report results from the MOVE-FA phase III study on vatiquinone in the second quarter of 2023
Zacks Rank & Stocks to Consider
Reatacurrently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector include eFFECTOR Therapeutics EFTR and 89BIO ETNB. While eFFECTOR Therapeutics sports a Zacks Rank #1 (Strong Buy), 89BIO has a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Loss estimates for eFFECTOR Therapeutics for 2023have narrowed from 96 cents to 88 cents in the past 60 days.
Earnings of eFFECTOR Therapeutics beat estimates in each of the trailing four quarters. The average earnings surprise for EFTR is 104.56%. EFTR stock has declined 88.9% in the past year. eFFECTOR Therapeutics is expected to report its fourth-quarter results next month.
Loss estimates for 89BIO for 2023 have narrowed from $2.67 per share to $2.59 per share in the past 60 days.
89BIO’s earnings beat estimates in three of the trailing four quarters, delivering an average earnings surprise of 10.08%. Shares of 89BIO have increased 249.1% in the past year. 89BIO is expected to report its fourth-quarter results next month.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
PTC Therapeutics, Inc. (PTCT) : Free Stock Analysis Report
Reata Pharmaceuticals, Inc. (RETA) : Free Stock Analysis Report
89BIO (ETNB) : Free Stock Analysis Report
eFFECTOR Therapeutics, Inc. (EFTR) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 