Replimune (REPL) Gains on Positive Data From Melanoma Study
Replimune Group, Inc. REPL announced positive top-line results from the primary analysis of the IGNYTE study on lead pipeline candidate RP1, in combination with Bristol Myers’ BMY blockbuster immuno-oncology drug Opdivo (nivolumab), in patients with anti-PD1 failed melanoma. The analysis was conducted by an independent central review.
RP1 is based on a proprietary strain of herpes simplex virus, engineered and genetically armed with a fusogenic protein (GALV-GP R-) and GM-CSF, intended to maximize tumor killing potency, the immunogenicity of tumor cell death and the activation of a systemic anti-tumor immune response.
The anti-PD1 failed melanoma cohort from the IGNYTE clinical trial included 140 patients. These patients received RP1 plus Opdivo after confirmed progression while being treated with at least eight weeks of prior anti-PD1 therapy. The primary analysis by independent central review was triggered once all patients had been followed for at least 12 months.
Results showed that one-third of patients receiving the combination of RP1 and nivolumab responded to treatment and improved upon the investigator-assessed data presented at ASCO 2024.
The overall response rate (ORR) was 33.6% by modified RECIST 1.1 criteria, the primary endpoint as defined in the protocol. ORR was 32.9% by RECIST 1.1 criteria, an additional analysis requested by the FDA. Responses from the baseline were highly durable, with all responses lasting more than six months and the median duration of response exceeding 35 months.
Data presented at the ASCO showed that approximately one-third of patients experienced a response, with an ORR by investigator assessment of 32.7%.
The combination continues to be well-tolerated, with mainly grade 1-2 constitutional-type side effects.
Shares of Replimune have surged 27.88% on the encouraging results.
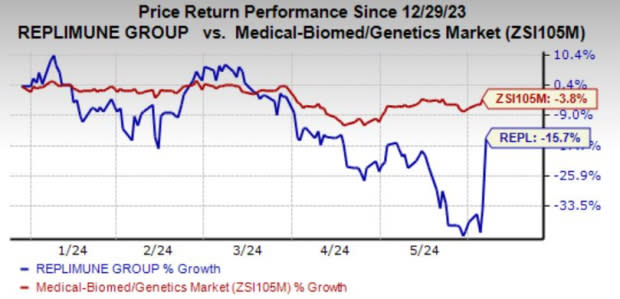
Image Source: Zacks Investment Research
In the year-to-date period, shares of REPL have lost 15.7% compared with the industry’s decline of 3.8%.
Replimune has shared these positive results with the FDA. The company plans to request a meeting with the agency before its intended biologics license application (BLA) submission. The BLA submission is targeted in the second half of 2024.
The first patient is expected to be enrolled in IGNYTE-3 confirmatory trial in the third quarter of 2024.
The company is also evaluating RP1 in solid organ transplant recipients with skin cancers. RPI, in combination with skin cancer drug Libtayo, is also being evaluated for the treatment of cutaneous squamous cell carcinoma.
Replimune’s proprietary RPx platform is based on a potent HSV-1 backbone, intended to maximize immunogenic cell death and the induction of a systemic anti-tumor immune response. The company currently has three RPx product candidates in its portfolio, namely RP1, RP2 and RP3.
RP2 is being evaluated for the treatment of uveal melanoma and hepatocellular carcinoma (HCC). The protocol for the registration-directed clinical trial on RP2, combined with Opdivo in advanced uveal melanoma, is near final following input from the FDA.
A mid-stage study evaluating RP2 in anti-PD1/PD-L1 progressed HCC of RP2, combined with atezolizumab and bevacizumab, is expected to be initiated in the second half of 2024.
The successful development of its candidates should be a significant boost for this clinical-stage biotechnology company.
BMY’s Opdivo is approved, both as a monotherapy and in combination with Yervoy, to treat a plethora of cancer indications in many countries, including the United States and the EU.
Zacks Rank & Stocks to Consider
Replimune currently carries a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the healthcare sector are Krystal Biotech, Inc. KRYS and ALX Oncology Holdings ALXO, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for KRYS’ earnings per share has increased 24 cents to $2.06. KRYS beat on earnings in two of the trailing four quarters and missed the mark in the other two, delivering an average negative surprise of 21.46%. Shares of Krystal Biotech have surged 33.6% year to date.
In the past 30 days, the Zacks Consensus Estimate for ALX Oncology’s 2024 loss per share has narrowed from $3.33 to $2.89. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.85 to $2.73.
ALX Oncology beat on earnings in two of the trailing four quarters and missed the mark in the other two, delivering an average negative surprise of 8.83%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Krystal Biotech, Inc. (KRYS) : Free Stock Analysis Report
Replimune Group, Inc. (REPL) : Free Stock Analysis Report
ALX Oncology Holdings Inc. (ALXO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 