Roche (RHHBY) Announces Positive Results From PNH Studies
Roche RHHBY announced positive results from the late-stage COMMODORE 1 and 2 studies on crovalimab for the treatment of paroxysmal nocturnal hemoglobinuria (PNH).
The COMMODORE 1 study is a phase III, randomized, open-label study evaluating the safety of crovalimab in people with PNH switching from currently approved C5 inhibitors.
The COMMODORE 2 study is a phase III, randomized, open-label study evaluating the efficacy and safety of crovalimab versus Soliris (a current standard of care for PNH) in people with PNH who have not been treated previously with C5 inhibitors.
PNH is a rare and life-threatening blood condition in which red blood cells are destroyed by the complement system — part of the innate immune system — causing symptoms such as anemia, fatigue, blood clots and kidney disease.
Crovalimab is an investigational, novel anti-C5 recycling monoclonal antibody. The C5 inhibitors are known to be effective in treating this condition.
Data from the COMMODORE 2 study showed 79.3% of participants randomized to be treated with crovalimab achieved hemolysis control from week five to week 25 compared with 79.0% with Soliris. Approximately 65.7% achieved transfusion avoidance (TA) from baseline to week 25 with crovalimab and 68.1% with Soliris. Blood transfusion requirements are important clinical measures of hemolysis caused by complement dysregulation in PNH. While the COMMODORE 2 study demonstrated that subcutaneous crovalimab every four weeks was non-inferior to intravenous Soliris every two weeks, with comparable safety, in people new to C5 inhibitors, the COMMODORE 1 study in people switching from currently approved C5 inhibitors supported the consistent benefit-risk profile of crovalimab as seen in the COMMODORE 2 study.
Adverse events occurred in 78% of participants treated with crovalimab and 80% treated with Soliris in the COMMODORE 2 study.
The results were presented at the European Hematology Association (EHA) Hybrid Congress.
Roche also presented preliminary data from the COMMODORE Burden of Illness study, suggesting that despite currently available C5 inhibitor treatments, people with PNH continue to bear a substantial burden of disease, which can translate into diminished quality of life and considerable costs.
Roche plans to submit global phase III data from the COMMODORE 1 and 2 studies in PNH to regulatory authorities around the world.
Earlier, positive data from a third phase III study evaluating crovalimab in PNH, the COMMODORE 3 study in China, were presented at the American Society of Hematology (ASH) Annual Meeting and Exposition in December 2022.
Crovalimab is also being evaluated for atypical hemolytic uremic syndrome, sickle cell disease, and other complement-mediated diseases.
The successful development of crovalimab will be a boost to Roche’s portfolio.
Roche’s stock has gained 1.5% in the past year compared with the industry’s growth of 15.1%.
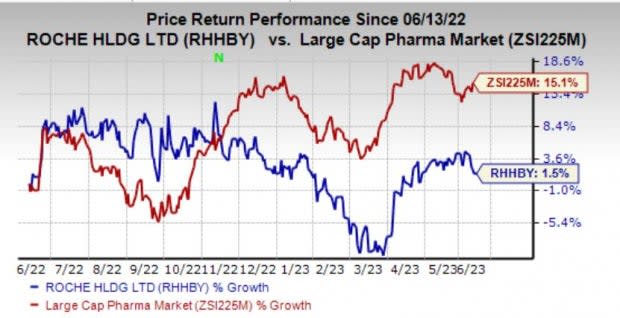
Image Source: Zacks Investment Research
Roche’s performance in the first quarter was ordinary as significantly lower COVID-19 product sales impacted the top line. However, new drugs — Ocrevus, Hemlibra, Evrysdi and Tecentriq — recorded growth and the uptake of the new eye drug, Vabysmo (launched at the beginning of 2022), was outstanding.
Sales are likely to be impacted further due to the expected sharp decline in sales of COVID-19 products of roughly CHF 5 billion in 2023. Competition from biosimilars for established cancer medicines like Avastin, MabThera/Rituxan and Herceptin also harmed sales.
Zacks Rank & Stocks to Consider
Roche currently carries a Zacks Rank #2 (Buy). Some other top-ranked stocks in the healthcare sector are Ligand Pharmaceuticals LGND and Novartis NVS. While Ligand sports a Zacks Rank #1 (Strong Buy), NVS carries a Zacks Rank #2. You can see the complete list of today’s Zacks #1 Rank stocks here.
Over the past 30 days, earnings estimates for LGND have increased by 46 cents per share to $5.25. LGND topped earnings estimates in two of the last four quarters and missed in the remaining two, the average surprise being 21.50%.
Over the past 60 days, NVS’ earnings estimates have increased to $6.72 from $6.57 for 2023. Novartis surpassed estimates in all the trailing four quarters, the average surprise being 5.15%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 